Immune Netw.
2017 Feb;17(1):20-24. 10.4110/in.2017.17.1.20.
Microbiota Influences Vaccine and Mucosal Adjuvant Efficacy
- Affiliations
-
- 1Division of Biochemistry, Faculty of Pharmacy, Keio University, Tokyo 105-8512, Japan. ykim@keio.jp
- KMID: 2368973
- DOI: http://doi.org/10.4110/in.2017.17.1.20
Abstract
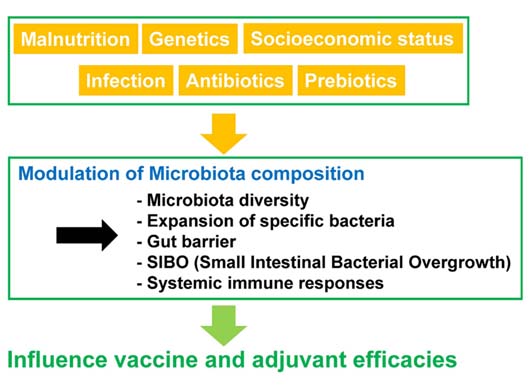
- A symbiotic relationship between humans and the microbiota is critical for the maintenance of our health, including development of the immune system, enhancement of the epithelial barrier, and acquisition of nutrients. Recent research has shown that the microbiota impacts immune cell development and differentiation. These findings suggest that the microbiota may also influence adjuvant and vaccine efficacy. Indeed, several factors such as malnutrition and poor sanitation, which affect gut microbiota composition, impair the efficacy of vaccines. Although there is little evidence that microbiota alters vaccine efficacy, further understanding of human immune system-microbiota interactions may lead to the effective development of adjuvants and vaccines for the treatment of diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013; 13:321–335.
Article2. McKenney PT, Pamer EG. From hype to hope: The gut microbiota in enteric infectious disease. Cell. 2015; 163:1326–1332.
Article3. Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 2016; 16:295–309.
Article4. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014; 157:121–141.
Article5. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009; 9:313–323.
Article6. Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005; 122:107–118.
Article7. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004; 303:1662–1665.
Article8. Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Nunez G. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity. 2016; 44:647–658.
Article9. Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008; 456:507–510.
Article10. Hansen CH, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T, Tlaskalova-Hogenova H, Hansen AK. Patterns of early gut colonization shape future immune responses of the host. PLoS One. 2012; 7:e34043.
Article11. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384.
Article12. Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev. 2015; 95:149–178.
Article13. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013; 54:2325–2340.
Article14. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003; 133:2485S–2493S.
Article15. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013; 504:446–450.
Article16. Vesikari T, Isolauri E, D'Hondt E, Delem A, Andre FE, Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984; 1:977–981.
Article17. John TJ, Jayabal P. Oral polio vaccination of children in the tropics. I. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol. 1972; 96:263–269.
Article18. Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991; 13:926–939.
Article19. Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin. 2010; 6:532–542.
Article20. Lopman BA, Pitzer VE, Sarkar R, Gladstone B, Patel M, Glasser J, Gambhir M, Atchison C, Grenfell BT, Edmunds WJ, Kang G, Parashar UD. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS One. 2012; 7:e41720.
Article21. Hallander HO, Paniagua M, Espinoza F, Askelof P, Corrales E, Ringman M, Storsaeter J. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine. 2002; 21:138–145.
Article22. Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, Aylward RB. Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J Infect Dis. 2009; 200:794–801.
Article23. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A . 2010; 107:14691–14696.
Article24. Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB. Stool microbiota and vaccine responses of infants. Pediatrics. 2014; 134:e362–e372.
Article25. Lagos R, Fasano A, Wasserman SS, Prado V, San Martin O, Abrego P, Losonsky GA, Alegria S, Levine MM. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J Infect Dis. 1999; 180:1709–1712.
Article26. Reikie BA, Adams RC, Ruck CE, Ho K, Leligdowicz A, Pillay S, Naidoo S, Fortuno ES III, de Beer C, Preiser W, Cotton MF, Speert DP, Esser M, Kollmann TR. Ontogeny of Toll-like receptor mediated cytokine responses of South African infants throughout the first year of life. PLoS One. 2012; 7:e44763.
Article27. Smolen KK, Ruck CE, Fortuno ES III, Ho K, Dimitriu P, Mohn WW, Speert DP, Cooper PJ, Esser M, Goetghebuer T, Marchant A, Kollmann TR. Pattern recognition receptor-mediated cytokine response in infants across 4 continents. J Allergy Clin Immunol. 2014; 133:818–826.
Article28. Woo PC, Tsoi HW, Wong LP, Leung HC, Yuen KY. Antibiotics modulate vaccine-induced humoral immune response. Clin Diagn Lab Immunol. 1999; 6:832–837.
Article29. Seekatz AM, Panda A, Rasko DA, Toapanta FR, Eloe-Fadrosh EA, Khan AQ, Liu Z, Shipley ST, Detolla LJ, Sztein MB, Fraser CM. Differential response of the cynomolgus macaque gut microbiota to Shigella infection. PLoS One. 2013; 8:e64212.
Article30. Benyacoub J, Rochat F, Saudan KY, Rochat I, Antille N, Cherbut C, von der Weid T, Schiffrin EJ, Blum S. Feeding a diet containing a fructooligosaccharide mix can enhance Salmonella vaccine efficacy in mice. J Nutr. 2008; 138:123–129.
Article31. Vos AP, Haarman M, Buco A, Govers M, Knol J, Garssen J, Stahl B, Boehm G, M'Rabet L. A specific prebiotic oligosaccharide mixture stimulates delayed-type hypersensitivity in a murine influenza vaccination model. Int Immunopharmacol. 2006; 6:1277–1286.
Article32. Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004; 82:488–496.
Article33. Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015; 32:21–27.
Article34. Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, McCausland M, Kanchan V, Kokko KE, Li S, Elbei R, Mehta AK, Aderem A, Subbarao K, Ahmed R, Pulendran B. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011; 12:786–795.
Article35. Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, Sartor RB, Gewirtz AT, Pulendran B. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014; 41:478–492.
Article36. Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992; 22:2277–2281.
Article37. Sunahara RK, Dessauer CW, Whisnant RE, Kleuss C, Gilman AG. Interaction of Gsalpha with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem. 1997; 272:22265–22271.
Article38. Kim D, Kim YG, Seo SU, Kim DJ, Kamada N, Prescott D, Philpott DJ, Rosenstiel P, Inohara N, Nunez G. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat Med. 2016; 22:524–530.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mucosal vaccine adjuvants update
- Influence of the Host Factors on Human Papillomavirus Infection and Vaccine Efficacy
- Tetanus toxin fragment C fused to flagellin makes a potent mucosal vaccine
- Influence of Immunity Induced at Priming Step on Mucosal Immunization of Heterologous Prime-Boost Regimens
- Recent progress in mucosal immunology and vaccine development