Mucosal vaccine adjuvants update
- Affiliations
-
- 1Clinical Vaccine R&D Center, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea. jhrhee@chonnam.ac.kr
- 2Department of Microbiology and Research Institute of Vibrio Infections, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Pharmacology and Dental Therapeutics, School of Dentistry, Chonnam National University, Gwangju, Korea.
- 4Fraunhofer Korea Center for Biopharmaceutical Research, Hwasun, Korea.
- KMID: 2278788
- DOI: http://doi.org/10.7774/cevr.2012.1.1.50
Abstract
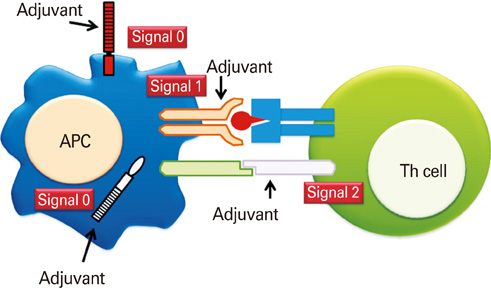
- Mucosal vaccination, capable of inducing protective immune responses both in the mucosal and systemic immune compartments, has many advantages and is regarded as a blue ocean in the vaccine industry. Mucosal vaccines can offer lower costs, better accessability, needle-free delivery, and higher capacity of mass immunizations during pandemics. However, only very limited number of mucosal vaccines was approved for human use in the market yet. Generally, induction of immune responses following mucosal immunization requires the co-administration of appropriate adjuvants that can initiate and support the effective collaboration between innate and adaptive immunity. Classically, adjuvant researches were rather empirical than keenly scientific. However, during last several years, fundamental scientific achievements in innate immunity have been translated into the development of new mucosal adjuvants. This review focuses on recent developments in the concepts of adjuvants and innate immunity, mucosal immunity with special interest of vaccine development, and basic and applied researches in mucosal adjuvant.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Enhancement of antigen-specific humoral immune responses and protein solubility through conjugation of bacterial flagellin, Vibrio vulnificus FlaB, to the N-terminus of porcine epidemic diarrhea virus surface protein antigen S0
Seo-ho Oh, Young-Saeng Kim Cho, Ho-Bin Lee, Sang-Mok Lee, Whee-Soo Kim, Liang Hong, Chong-Su Cho, Yun-Jaie Choi, Sang-Kee Kang
J Vet Sci. 2019;20(6):. doi: 10.4142/jvs.2019.20.e70.Intranasal immunization with a flagellin-adjuvanted peptide anticancer vaccine prevents tumor development by enhancing specific cytotoxic T lymphocyte response in a mouse model
Chung Truong Nguyen, Seol Hee Hong, Thuan Trong Ung, Vivek Verma, Soo Young Kim, Joon Haeng Rhee, Shee Eun Lee
Clin Exp Vaccine Res. 2013;2(2):128-134. doi: 10.7774/cevr.2013.2.2.128.Towards Vaccine 3.0: new era opened in vaccine research and industry
Joon Haeng Rhee
Clin Exp Vaccine Res. 2014;3(1):1-4. doi: 10.7774/cevr.2014.3.1.1.Tetanus toxin fragment C fused to flagellin makes a potent mucosal vaccine
Shee Eun Lee, Chung Truong Nguyen, Soo Young Kim, Thinh Nguyen Thi, Joon Haeng Rhee
Clin Exp Vaccine Res. 2015;4(1):59-67. doi: 10.7774/cevr.2015.4.1.59.
Reference
-
1. Chen W, Patel GB, Yan H, Zhang J. Recent advances in the development of novel mucosal adjuvants and antigen delivery systems. Hum Vaccin. 2010. 6:706–714.
Article2. Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997. 51:311–340.
Article3. Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006. 6:148–158.
Article4. Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003. 21:Suppl 2. S89–S95.
Article5. Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001. 344:564–572.
Article6. Mutsch M, Zhou W, Rhodes P, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004. 350:896–903.
Article7. Fujkuyama Y, Tokuhara D, Kataoka K, et al. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines. 2012. 11:367–379.
Article8. Ramon G. Sur l'augmentation anormale de l'antitoxine chez les chevaux producteurs de serum antidiphtherique. Bull Soc Cent Med Vet. 1925. 101:227–234.9. Glenny AT. Insoluble precipitates in diphtheria and tetanus immunization. Br Med J. 1930. 2:244–245.
Article10. Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003. 8:934–943.
Article11. Schijns VE. Induction and direction of immune responses by vaccine adjuvants. Crit Rev Immunol. 2001. 21:75–85.
Article12. Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007. 5:505–517.
Article13. McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007. 27:687–690.
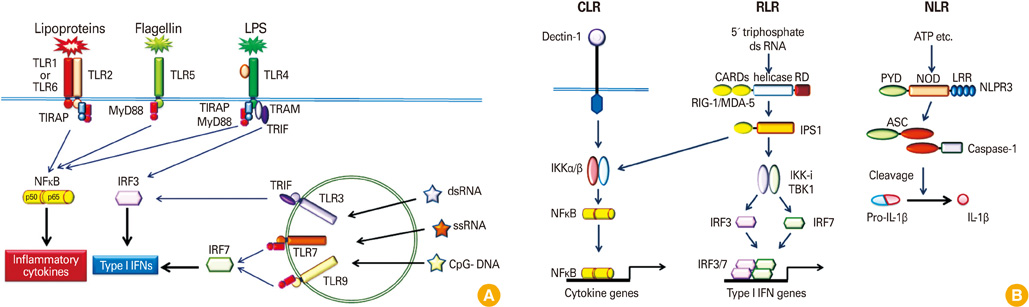
Article14. Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006. 27:352–357.
Article15. Akira S. Innate immunity and adjuvants. Philos Trans R Soc Lond B Biol Sci. 2011. 366:2748–2755.
Article16. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996. 86:973–983.
Article17. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006. 124:783–801.
Article18. Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007. 449:819–826.
Article19. Shi Z, Cai Z, Sanchez A, et al. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J Biol Chem. 2011. 286:4517–4524.
Article20. Flacher V, Bouschbacher M, Verronèse E, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006. 177:7959–7967.
Article21. Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001. 31:3388–3393.
Article22. Schwarz TF. Clinical update of the AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine, Cervarix. Adv Ther. 2009. 26:983–998.
Article23. Didierlaurent AM, Morel S, Lockman L, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009. 183:6186–6197.
Article24. Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009. 61:195–204.
Article25. Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011. 239:178–196.
Article26. Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006. 7:1250–1257.
Article27. Inohara , Chamaillard , McDonald C, Nuñez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005. 74:355–383.
Article28. Traub S, von Aulock S, Hartung T, Hermann C. MDP and other muropeptides: direct and synergistic effects on the immune system. J Endotoxin Res. 2006. 12:69–85.
Article29. Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006. 442:39–44.
Article30. Schroder K, Tschopp J. The inflammasomes. Cell. 2010. 140:821–832.
Article31. Holt LB. Quantitative studies in diphtheria prophylaxis: the second response. Br J Exp Pathol. 1950. 31:233–241.32. Iyer S, HogenEsch H, Hem SL. Relationship between the degree of antigen adsorption to aluminum hydroxide adjuvant in interstitial fluid and antibody production. Vaccine. 2003. 21:1219–1223.
Article33. Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007. 178:5271–5276.
Article34. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008. 453:1122–1126.
Article35. Kool M, Pétrilli V, De Smedt T, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008. 181:3755–3759.
Article36. Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008. 9:847–856.
Article37. Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008. 38:2085–2089.
Article38. Matzinger P. The danger model: a renewed sense of self. Science. 2002. 296:301–305.
Article39. Spreafico R, Ricciardi-Castagnoli P, Mortellaro A. The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. Eur J Immunol. 2010. 40:638–642.
Article40. Sharp FA, Ruane D, Claass B, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009. 106:870–875.
Article41. Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008. 181:17–21.
Article42. Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009. 227:54–65.
Article43. Yoneyama M, Kikuchi M, Natsukawa T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004. 5:730–737.
Article44. Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009. 227:75–86.
Article45. Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006. 441:101–105.
Article46. Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009. 461:788–792.
Article47. Takaoka A, Wang Z, Choi MK, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007. 448:501–505.
Article48. Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012. 12:125–135.
Article49. Lavelle EC, Murphy C, O'Neill LA, Creagh EM. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010. 3:17–28.
Article50. Varol C, Vallon-Eberhard A, Elinav E, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009. 31:502–512.
Article51. Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007. 204:1757–1764.
Article52. Kiyono H, Fukuyama S. NALT-versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004. 4:699–710.53. Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003. 13:293–310.
Article54. Kim DY, Sato A, Fukuyama S, et al. The airway antigen sampling system: respiratory M cells as an alternative gateway for inhaled antigens. J Immunol. 2011. 186:4253–4262.
Article55. Yamamoto M, Pascual DW, Kiyono H. M cell-targeted mucosal vaccine strategies. Curr Top Microbiol Immunol. 2012. 354:39–52.
Article56. Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010. 33:479–491.
Article57. Kunisawa J, Nochi T, Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends Immunol. 2008. 29:505–513.
Article58. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005. 11:4 Suppl. S45–S53.
Article59. Lawson LB, Norton EB, Clements JD. Defending the mucosa: adjuvant and carrier formulations for mucosal immunity. Curr Opin Immunol. 2011. 23:414–420.
Article60. Mishra N, Goyal AK, Tiwari S, et al. Recent advances in mucosal delivery of vaccines: role of mucoadhesive/biodegradable polymeric carriers. Expert Opin Ther Pat. 2010. 20:661–679.
Article61. Pavot V, Rochereau N, Genin C, Verrier B, Paul S. New insights in mucosal vaccine development. Vaccine. 2012. 30:142–154.
Article62. Eriksson K, Holmgren J. Recent advances in mucosal vaccines and adjuvants. Curr Opin Immunol. 2002. 14:666–672.
Article63. Jabbal-Gill I, Watts P, Smith A. Chitosan-based delivery systems for mucosal vaccines. Expert Opin Drug Deliv. 2012. 06. 19. [Epub] http://dx.doi.org/10.1517/17425247.2012.697455.
Article64. Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005. 23:1804–1813.
Article65. Bracho G, Lastre M, del Campo J, et al. Proteoliposome derived cochleate as novel adjuvant. Vaccine. 2006. 24:Suppl 2. S2-30–S2-31.
Article66. Cox E, Verdonck F, Vanrompay D, Goddeeris B. Adjuvants modulating mucosal immune responses or directing systemic responses towards the mucosa. Vet Res. 2006. 37:511–539.
Article67. Coulter A, Harris R, Davis R, et al. Intranasal vaccination with ISCOMATRIX adjuvanted influenza vaccine. Vaccine. 2003. 21:946–949.
Article68. Sanders MT, Deliyannis G, Pearse MJ, McNamara MK, Brown LE. Single dose intranasal immunization with ISCOMATRIX vaccines to elicit antibody-mediated clearance of influenza virus requires delivery to the lower respiratory tract. Vaccine. 2009. 27:2475–2482.
Article69. Vujanic A, Snibson KJ, Wee JL, et al. Long-term antibody and immune memory response induced by pulmonary delivery of the influenza Iscomatrix vaccine. Clin Vaccine Immunol. 2012. 19:79–83.
Article70. Lee SE, Kim SY, Jeong BC, et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006. 74:694–702.
Article71. Nguyen CT, Kim SY, Kim MS, Lee SE, Rhee JH. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine. 2011. 29:5731–5739.
Article72. Hong SH, Byun YH, Nguyen CT, et al. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine. 2012. 30:466–474.
Article73. Chen W, Kuolee R, Yan H. The potential of 3',5'-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine. 2010. 28:3080–3085.
Article74. Yan H, KuoLee R, Tram K, et al. 3',5'-Cyclic diguanylic acid elicits mucosal immunity against bacterial infection. Biochem Biophys Res Commun. 2009. 387:581–584.
Article75. Mowat AM, Smith RE, Donachie AM, Furrie E, Grdic D, Lycke N. Oral vaccination with immune stimulating complexes. Immunol Lett. 1999. 65:133–140.
Article76. Noda K, Kodama S, Umemoto S, Abe N, Hirano T, Suzuki M. Nasal vaccination with P6 outer membrane protein and alpha-galactosylceramide induces nontypeable Haemophilus influenzae-specific protective immunity associated with NKT cell activation and dendritic cell expansion in nasopharynx. Vaccine. 2010. 28:5068–5074.
Article77. Lee YS, Lee KA, Lee JY, et al. An alpha-GalCer analogue with branched acyl chain enhances protective immune responses in a nasal influenza vaccine. Vaccine. 2011. 29:417–425.
Article78. Courtney AN, Nehete PN, Nehete BP, Thapa P, Zhou D, Sastry KJ. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine. 2009. 27:3335–3341.
Article79. Lindqvist M, Persson J, Thorn K, Harandi AM. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J Immunol. 2009. 182:6435–6443.
Article80. de Haan L, Hirst TR. Cholera toxin and related enterotoxins: a cell biological and immunological perspective. J Nat Toxins. 2000. 9:281–297.81. van Ginkel FW, Jackson RJ, Yoshino N, et al. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect Immun. 2005. 73:6892–6902.
Article82. Eriksson K, Fredriksson M, Nordström I, Holmgren J. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect Immun. 2003. 71:1740–1747.
Article83. George-Chandy A, Eriksson K, Lebens M, Nordström I, Schön E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect Immun. 2001. 69:5716–5725.
Article84. Pizza M, Giuliani MM, Fontana MR, et al. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001. 19:2534–2541.
Article85. Mowat AM, Donachie AM, Jagewall S, et al. CTA1-DD-immune stimulating complexes: a novel, rationally designed combined mucosal vaccine adjuvant effective with nanogram doses of antigen. J Immunol. 2001. 167:3398–3405.
Article86. Sanchez J, Wallerstrom G, Fredriksson M, Angstrom J, Holmgren J. Detoxification of cholera toxin without removal of its immunoadjuvanticity by the addition of (STa-related) peptides to the catalytic subunit. A potential new strategy to generate immunostimulants for vaccination. J Biol Chem. 2002. 277:33369–33377.87. Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol. 2011. 18:546–551.
Article88. Anosova NG, Chabot S, Shreedhar V, Borawski JA, Dickinson BL, Neutra MR. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer's patches. Mucosal Immunol. 2008. 1:59–67.
Article89. Fahlen-Yrlid L, Gustafsson T, Westlund J, et al. CD11c (high) dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J Immunol. 2009. 183:5032–5041.
Article90. Chang SY, Cha HR, Igarashi O, et al. Cutting edge: Langerin+ dendritic cells in the mesenteric lymph node set the stage for skin and gut immune system cross-talk. J Immunol. 2008. 180:4361–4365.
Article91. Datta SK, Sabet M, Nguyen KP, et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc Natl Acad Sci U S A. 2010. 107:10638–10643.
Article92. Lu YJ, Yadav P, Clements JD, et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol. 2010. 17:1005–1012.
Article93. Meza-Sanchez D, Perez-Montesinos G, Sanchez-Garcia J, Moreno J, Bonifaz LC. Intradermal immunization in the ear with cholera toxin and its non-toxic beta subunit promotes efficient Th1 and Th17 differentiation dependent on migrating DCs. Eur J Immunol. 2011. 41:2894–2904.94. Raghavan S, Ostberg AK, Flach CF, et al. Sublingual immunization protects against Helicobacter pylori infection and induces T and B cell responses in the stomach. Infect Immun. 2010. 78:4251–4260.
Article95. Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008. 226:160–171.
Article96. Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009. 374:301–314.97. Schwarz TF, Spaczynski M, Schneider A, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009. 27:581–587.
Article98. Cranage MP, Fraser CA, Cope A, et al. Antibody responses after intravaginal immunisation with trimeric HIV-1 CN54 clade C gp140 in Carbopol gel are augmented by systemic priming or boosting with an adjuvanted formulation. Vaccine. 2011. 29:1421–1430.
Article99. Huang CF, Wu TC, Chu YH, Hwang KS, Wang CC, Peng HJ. Effect of neonatal sublingual vaccination with native or denatured ovalbumin and adjuvant CpG or cholera toxin on systemic and mucosal immunity in mice. Scand J Immunol. 2008. 68:502–510.
Article100. Pesce I, Monaci E, Muzzi A, et al. Intranasal administration of CpG induces a rapid and transient cytokine response followed by dendritic and natural killer cell activation and recruitment in the mouse lung. J Innate Immun. 2010. 2:144–159.
Article101. Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011. 10:499–511.
Article102. Samatey FA, Imada K, Nagashima S, et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature. 2001. 410:331–337.
Article103. Ramos HC, Rumbo M, Sirard JC. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 2004. 12:509–517.
Article104. Staats HF, Bradney CP, Gwinn WM, et al. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J Immunol. 2001. 167:5386–5394.
Article105. Eo SK, Lee S, Kumaraguru U, Rouse BT. Immunopotentiation of DNA vaccine against herpes simplex virus via co-delivery of plasmid DNA expressing CCR7 ligands. Vaccine. 2001. 19:4685–4693.
Article106. Lillard JW Jr, Boyaka PN, Taub DD, McGhee JR. RANTES potentiates antigen-specific mucosal immune responses. J Immunol. 2001. 166:162–169.
Article107. Thompson AL, Johnson BT, Sempowski GD, et al. Maximal adjuvant activity of nasally delivered IL-1alpha requires adjuvant-responsive CD11c(+) cells and does not correlate with adjuvant-induced in vivo cytokine production. J Immunol. 2012. 188:2834–2846.
Article108. Agrawal S, Gupta S, Agrawal A. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS One. 2010. 5:e13418.
Article109. McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009. 27:3544–3552.
Article110. Merluzzi S, Frossi B, Gri G, Parusso S, Tripodo C, Pucillo C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood. 2010. 115:2810–2817.
Article111. Hu KF, Lovgren-Bengtsson K, Morein B. Immunostimulating complexes (ISCOMs) for nasal vaccination. Adv Drug Deliv Rev. 2001. 51:149–159.
Article112. Kersten G, Hirschberg H. Antigen delivery systems. Expert Rev Vaccines. 2004. 3:453–462.
Article113. Romling U, Amikam D. Cyclic di-GMP as a second messenger. Curr Opin Microbiol. 2006. 9:218–228.
Article114. Pedersen GK, Ebensen T, Gjeraker IH, et al. Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PLoS One. 2011. 6:e26973.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants
- Development of mucosal vaccine delivery: an overview on the mucosal vaccines and their adjuvants
- Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease
- Recent Advances of Vaccine Adjuvants for Infectious Diseases
- Mucosal Immune System and M Cell-targeting Strategies for Oral Mucosal Vaccination