Cancer Res Treat.
2017 Jan;49(1):193-203. 10.4143/crt.2015.473.
Concurrent Chemoradiotherapy with Temozolomide Followed by Adjuvant Temozolomide for Newly Diagnosed Glioblastoma Patients: A Retrospective Multicenter Observation Study in Korea
- Affiliations
-
- 1Department of Neurosurgery, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
- 2Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Radiation Oncology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 5Division of Hematology/Oncology, Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Neurosurgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 7Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 8Department of Neurosurgery, Brain Tumor Center, Yonsei University Health System, Seoul, Korea.
- 9Department of Radiation Oncology, Brain Tumor Center, Yonsei University Health System, Seoul, Korea.
- 10Department of Neurosurgery, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 11Department of Pathology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 12Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 13Department of Radiation Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 14Department of Neurosurgery, Gangnam Severance Hospital, Yonsei University College of Health Science, Seoul, Korea.
- 15Department of Neuro-Oncology Clinic, Center for Specific Organs Cancer, National Cancer Center Hospital, National Cancer Center, Goyang, Korea.
- 16Department of Radiation Oncology, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 17Department of Neurosurgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 18Department of Radiation Oncology, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea.
- 19Department of Neurosurgery, Inha University Hospital, Inha University School of Medicine, Incheon, Korea.
- 20Department of Neurosurgery, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
- 21Department of Neurosurgery, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea.
- 22Department of Neurosurgery, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- 23Department of Neurosurgery, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 24Department of Neurosurgery, Soonchunhyang University Hospital, Seoul, Korea.
- 25Department of Neurosurgery, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 26Department of Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 27Department of Radiation, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 28Department of Pathology, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul, Korea.
- 29Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 30Department of Systemic Cancer Science, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea. nsghs@ncc.re.kr
- 31Division of Hematotology/Oncology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sehoon.lee119@gmail.com
- 32Department of Neurosurgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2367517
- DOI: http://doi.org/10.4143/crt.2015.473
Abstract
- PURPOSE
The purpose of this study was to investigate the feasibility and survival benefits of combined treatment with radiotherapy and adjuvant temozolomide (TMZ) in a Korean sample.
MATERIALS AND METHODS
A total of 750 Korean patients with histologically confirmed glioblastoma multiforme, who received concurrent chemoradiotherapy with TMZ (CCRT) and adjuvant TMZ from January 2006 until June 2011, were analyzed retrospectively.
RESULTS
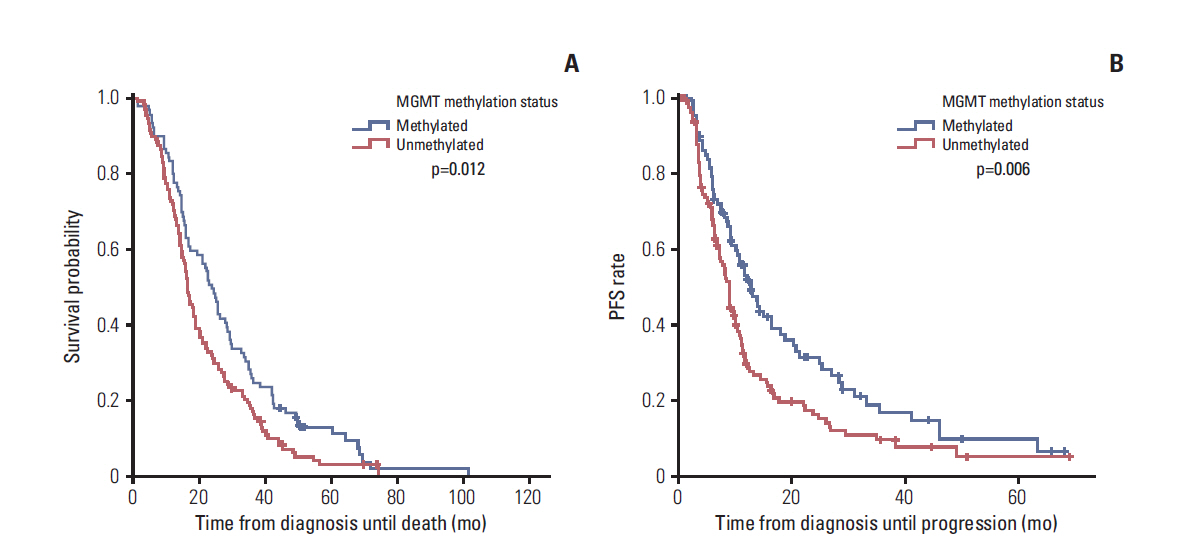
After the first operation, a gross total resection (GTR), subtotal resection (STR), partial resection (PR), biopsy alone were achieved in 388 (51.7%), 159 (21.2%), 96 (12.8%), and 107 (14.3%) patients, respectively. The methylation status of O6-methylguanine-DNA methyltransferase (MGMT) was reviewed retrospectively in 217 patients. The median follow-up period was 16.3 months and the median overall survival (OS) was 17.5 months. The actuarial survival rates at the 1-, 3-, and 5-year OS were 72.1%, 21.0%, and 9.0%, respectively. The median progression-free survival (PFS) was 10.1 months, and the actuarial PFS at 1-, 3-, and 5-year PFS were 42.2%, 13.0%, and 7.8%, respectively. The patients who received GTR showed a significantly longer OS and PFS than those who received STR, PR, or biopsy alone, regardless of the methylation status of the MGMT promoter. Patients with a methylated MGMT promoter also showed a significantly longer OS and PFS than those with an unmethylated MGMT promoter. Patients who received more than six cycles of adjuvant TMZ had a longer OS and PFS than those who received six or fewer cycles. Hematologic toxicity of grade 3 or 4 was observed in 8.4% of patients during the CCRT period and in 10.2% during the adjuvant TMZ period.
CONCLUSION
Patients treated with CCRT followed by adjuvant TMZ had more favorable survival rates and tolerable toxicity than those who did not undergo this treatment.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Dynamic Susceptibility Contrast (DSC) Perfusion MR in the Prediction of Long-Term Survival of Glioblastomas (GBM): Correlation with MGMT Promoter Methylation and 1p/19q Deletions
Yong Wonn Kwon, Won-Jin Moon, Mina Park, Hong Gee Roh, Young Cho Koh, Sang Woo Song, Jin Woo Choi
Investig Magn Reson Imaging. 2018;22(3):158-167. doi: 10.13104/imri.2018.22.3.158.Risk Factors for Cognitive Impairment in High-Grade Glioma Patients Treated with Postoperative Radiochemotherapy
Qiang Wang, Fengxia Xiao, Fei Qi, Xiaopeng Song, Yonghua Yu
Cancer Res Treat. 2020;52(2):586-593. doi: 10.4143/crt.2019.242.
Reference
-
References
1. Suzuki Y, Shirai K, Oka K, Mobaraki A, Yoshida Y, Noda SE, et al. Higher pAkt expression predicts a significant worse prognosis in glioblastomas. J Radiat Res. 2010; 51:343–8.
Article2. DeAngelis LM. Brain tumors. N Engl J Med. 2001; 344:114–23.
Article3. Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003; 30(6 Suppl 19):10–4.
Article4. Curran WJ Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993; 85:704–10.
Article5. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–96.
Article6. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–66.
Article7. Yang H, Wei D, Yang K, Tang W, Luo Y, Zhang J. The prognosis of MGMT promoter methylation in glioblastoma patients of different race: a meta-analysis. Neurochem Res. 2014; 39:2277–87.
Article8. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neurooncology working group. J Clin Oncol. 2010; 28:1963–72.
Article9. Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004; 6:227–35.10. Oike T, Suzuki Y, Sugawara K, Shirai K, Noda SE, Tamaki T, et al. Radiotherapy plus concomitant adjuvant temozolomide for glioblastoma: Japanese mono-institutional results. PLoS One. 2013; 8:e78943.
Article11. Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011; 12:997–1003.
Article12. Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008; 62:564–76.13. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001; 95:190–8.
Article14. Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003; 99:467–73.
Article15. Kreth FW, Thon N, Simon M, Westphal M, Schackert G, Nikkhah G, et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013; 24:3117–23.
Article16. Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999; 59:793–7.17. Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, et al. Inactivation of the DNArepair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000; 343:1350–4.18. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352:997–1003.19. Rivera AL, Pelloski CE, Gilbert MR, Colman H, De La Cruz C, Sulman EP, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010; 12:116–21.
Article20. Criniere E, Kaloshi G, Laigle-Donadey F, Lejeune J, Auger N, Benouaich-Amiel A, et al. MGMT prognostic impact on glioblastoma is dependent on therapeutic modalities. J Neurooncol. 2007; 83:173–9.
Article21. Lawrence YR, Blumenthal DT, Matceyevsky D, Kanner AA, Bokstein F, Corn BW. Delayed initiation of radiotherapy for glioblastoma: how important is it to push to the front (or the back) of the line? J Neurooncol. 2011; 105:1–7.
Article22. Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol. 2007; 85:339–43.
Article23. Seiz M, Krafft U, Freyschlag CF, Weiss C, Schmieder K, Lohr F, et al. Long-term adjuvant administration of temozolomide in patients with glioblastoma multiforme: experience of a single institution. J Cancer Res Clin Oncol. 2010; 136:1691–5.
Article24. Joo JD, Chang JH, Kim JH, Hong YK, Kim YH, Kim CY. Temozolomide during and after radiotherapy for newly diagnosed glioblastomas: a prospective multicenter study of Korean patients. J Korean Neurosurg Soc. 2012; 52:92–7.25. Park CK, Lee SH, Kim TM, Choi SH, Park SH, Heo DS, et al. The value of temozolomide in combination with radiotherapy during standard treatment for newly diagnosed glioblastoma. J Neurooncol. 2013; 112:277–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validation of the Effectiveness and Safety of Temozolomide during and after Radiotherapy for Newly Diagnosed Glioblastomas: 10-year Experience of a Single Institution
- Temozolomide during and after Radiotherapy for Newly Diagnosed Glioblastomas : A Prospective Multicenter Study of Korean Patients
- Glioblastoma in a Patient with Neurofibromatosis Type 1: A Case Report and Review of the Literature
- Temozolomide Drives Ferroptosis via a DMT1-Dependent Pathway in Glioblastoma Cells
- The Outcomes of Concomitant Chemoradiotherapy Followed by Adjuvant Chemotherapy with Temozolomide for Newly Diagnosed High Grade Gliomas : The Preliminary Results of Single Center Prospective Study