Validation of the Effectiveness and Safety of Temozolomide during and after Radiotherapy for Newly Diagnosed Glioblastomas: 10-year Experience of a Single Institution
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Seongnam, Korea. chaeyong@snu.ac.kr
- 2Department of Neurosurgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2351109
- DOI: http://doi.org/10.3346/jkms.2015.30.11.1597
Abstract
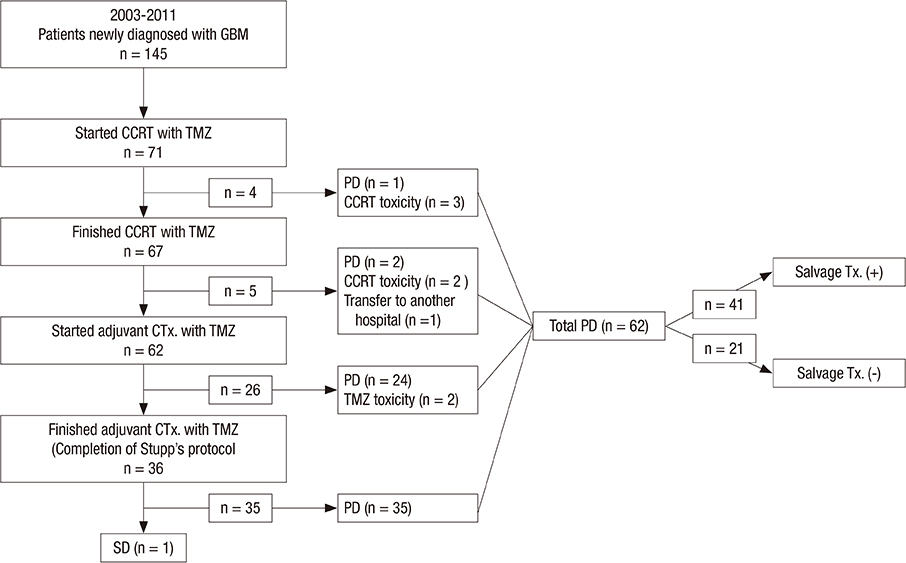
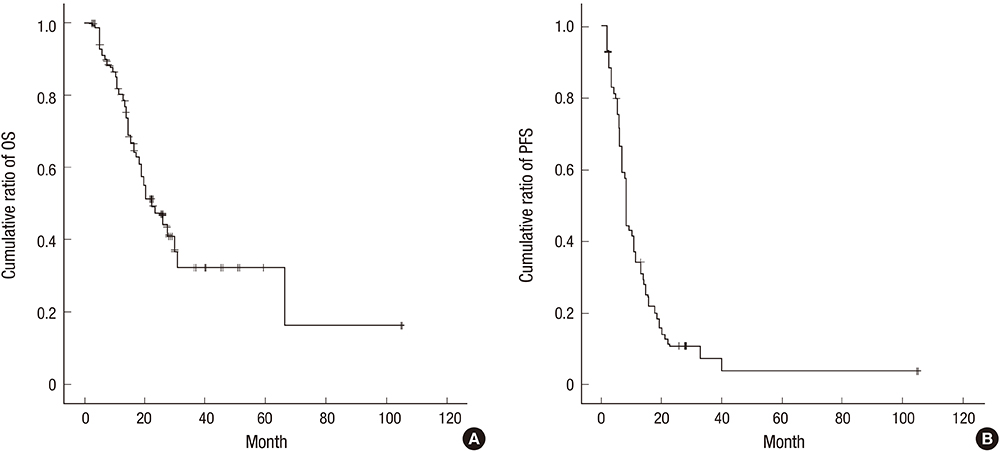
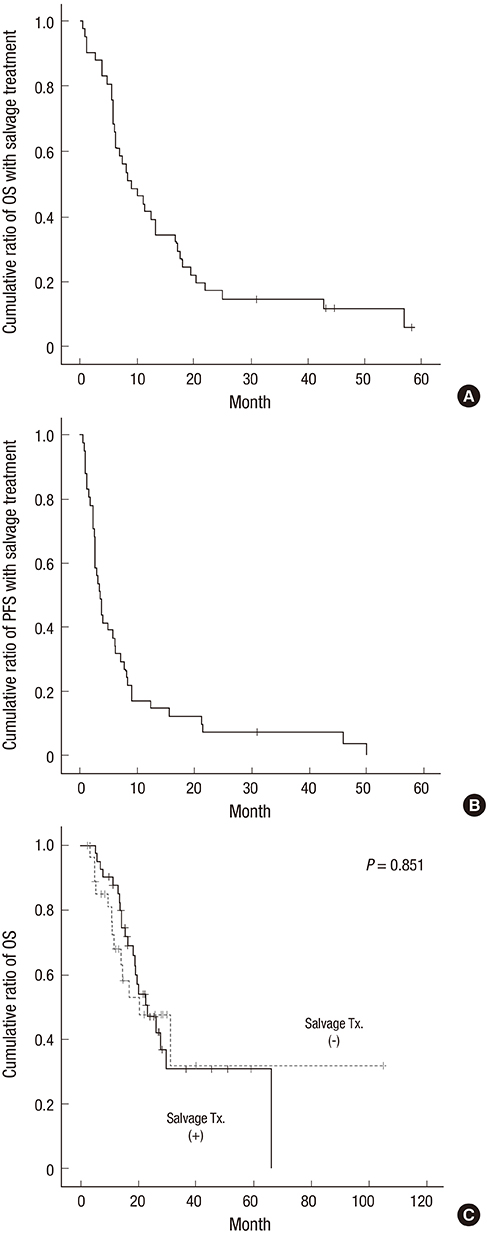
- This study was performed to validate the effectiveness and safety of concurrent chemoradiotherapy and adjuvant therapy with temozolomide for newly diagnosed glioblastoma multiforme as a standard treatment protocol. Between 2004 and 2011, patients newly diagnosed with glioblastoma who were treated with temozolomide during concurrent chemoradiotherapy and adjuvant chemotherapy were included from a single institution and analyzed retrospectively. The primary endpoint was overall survival, and the secondary endpoints were progression-free survival, response, and safety. A total of 71 patients were enrolled in this study. The response rate was 41% (29/71), and the tumor control rate was 80% (57/71). In the 67 patients who completed the concurrent chemoradiotherapy with temozolomide, the median overall survival was 19 months and the 1- and 2-yr overall survival rates were 78.3% and 41.7%, respectively. The median progression free survival was 9 months, and the 1- and 2-yr progression free survival rates were 33.8% and 14.3%, respectively. The mean duration of survival after progression of disease in salvage treatment group was 11.9 (1.3-53.2) months. Concurrent chemoradiotherapy with temozolomide resulted in grade 3 or 4 hematologic toxic effects in 2.8% of the patients. The current protocol of temozolomide during and after radiation therapy is both effective and safe and is still appropriate as the standard protocol for treatment of glioblastoma. An active salvage treatment might be required for a better prognosis.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Antineoplastic Agents, Alkylating/administration & dosage
Brain Neoplasms/diagnosis/*mortality/*therapy
Chemoradiotherapy, Adjuvant/methods/mortality
Comorbidity
Dacarbazine/administration & dosage/*analogs & derivatives
Female
Glioblastoma/diagnosis/*mortality/*therapy
Hematologic Diseases/*mortality
Humans
Longitudinal Studies
Male
Middle Aged
Prevalence
Radiotherapy, Conformal/mortality
Republic of Korea/epidemiology
Risk Factors
Survival Rate
Treatment Outcome
Young Adult
Antineoplastic Agents, Alkylating
Dacarbazine
Figure
Cited by 3 articles
-
Anti-Migration and Anti-Invasion Effects of Curcumin via Suppression of Fascin Expression in Glioblastoma Cells
Ki-Su Park, Sang-Youl Yoon, Seong-Hyun Park, Jeong-Hyun Hwang
Brain Tumor Res Treat. 2019;7(1):16-24. doi: 10.14791/btrt.2019.7.e28.Influence of Concurrent and Adjuvant Temozolomide on Health-Related Quality of Life of Patients with Grade III Gliomas: A Secondary Analysis of a Randomized Clinical Trial (KNOG-1101 Study)
Grace S. Ahn, Kihwan Hwang, Tae Min Kim, Chul Kee Park, Jong Hee Chang, Tae-Young Jung, Jin Hee Kim, Do-Hyun Nam, Se-Hyuk Kim, Heon Yoo, Yong-Kil Hong, Eun-Young Kim, Dong-Eun Lee, Jungnam Joo, Yu Jung Kim, Gheeyoung Choe, Byung Se Choi, Seok-Gu Kang, Jeong Hoon Kim, Chae-Yong Kim
Cancer Res Treat. 2022;54(2):396-405. doi: 10.4143/crt.2021.393.The Role of Early and Delayed Surgery for Infants with Congenital Brain Tumors
Jong Seok Lee, Ji Yeoun Lee, Kyung Hyun Kim, Sung-Hye Park, Eun Jung Koh, Seung-Ki Kim, Ji Hoon Phi
Cancer Res Treat. 2024;56(3):909-919. doi: 10.4143/crt.2023.1174.
Reference
-
1. Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003; 30:10–14.2. DeAngelis LM. Brain tumors. N Engl J Med. 2001; 344:114–123.3. Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009; 472:323–342.4. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.5. Choi JW, Lee MM, Kim IA, Kim JH, Choe G, Kim CY. The outcomes of concomitant chemoradiotherapy followed by adjuvant chemotherapy with temozolomide for newly diagnosed high grade gliomas: the preliminary results of single center prospective study. J Korean Neurosurg Soc. 2008; 44:222–227.6. Joo JD, Chang JH, Kim JH, Hong YK, Kim YH, Kim CY. Temozolomide during and after radiotherapy for newly diagnosed glioblastomas: a prospective multicenter study of korean patients. J Korean Neurosurg Soc. 2012; 52:92–97.7. Stupp R, Tonn JC, Brada M, Pentheroudakis G. Esmo Guidelines Working Group. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21:v190–v193.8. Kong DS, Lee JI, Kim JH, Kim ST, Kim WS, Suh YL, Dong SM, Nam DH. Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro Oncol. 2010; 12:289–296.9. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280.10. Chibbaro S, Benvenuti L, Caprio A, Carnesecchi S, Pulerà F, Faggionato F, Serino D, Galli C, Andreuccetti M, Buxton N, et al. Temozolomide as first-line agent in treating high-grade gliomas: phase II study. J Neurooncol. 2004; 67:77–81.11. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28:1963–1972.12. Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001; 12:259–266.13. Kim YH, Park CK, Cho WH, Kim IA, Moon S, Choe G, Park SH, Kim IH, Kim DG, Jung HW, et al. Temozolomide during and after radiation therapy for WHO grade III gliomas: preliminary report of a prospective multicenter study. J Neurooncol. 2011; 103:503–512.14. Friedman HS, McLendon RE, Kerby T, Dugan M, Bigner SH, Henry AJ, Ashley DM, Krischer J, Lovell S, Rasheed K, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. J Clin Oncol. 1998; 16:3851–3857.15. Ishikawa E, Yamamoto T, Sakamoto N, Nakai K, Akutsu H, Tsuboi K, Takano S, Matsumura A. Low peripheral lymphocyte count before focal radiotherapy plus concomitant temozolomide predicts severe lymphopenia during malignant glioma treatment. Neurol Med Chir (Tokyo). 2010; 50:638–644.16. Siker ML, Chakravarti A, Mehta MP. Should concomitant and adjuvant treatment with temozolomide be used as standard therapy in patients with anaplastic glioma? Crit Rev Oncol Hematol. 2006; 60:99–111.17. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466.18. Kesari S, Schiff D, Drappatz J, LaFrankie D, Doherty L, Macklin EA, Muzikansky A, Santagata S, Ligon KL, Norden AD, et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res. 2009; 15:330–337.19. Lakomý R, Fadrus P, Slampa P, Svoboda T, Kren L, Lzicarová E, Belanová R, Siková I, Poprach A, Schneiderová M, et al. Multimodal treatment of glioblastoma multiforme: results of 86 consecutive patients diagnosed in period 2003-2009. Klin Onkol. 2011; 24:112–120.20. Bae SH, Park MJ, Lee MM, Kim TM, Lee SH, Cho SY, Kim YH, Kim YJ, Park CK, Kim CY. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J Korean Med Sci. 2014; 29:980–984.21. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011; 155:39–51.22. Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010; 96:259–269.23. Hau P, Baumgart U, Pfeifer K, Bock A, Jauch T, Dietrich J, Fabel K, Grauer O, Wismeth C, Klinkhammer-Schalke M, et al. Salvage therapy in patients with glioblastoma: is there any benefit? Cancer. 2003; 98:2678–2686.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Temozolomide during and after Radiotherapy for Newly Diagnosed Glioblastomas : A Prospective Multicenter Study of Korean Patients
- Radiotherapy for Newly Diagnosed Glioblastoma in the Elderly: What Is the Standard?
- Overcoming Treatment Resistance in High Grade Gliomas
- Lynch Syndrome-Associated Glioblastoma Treated With Concomitant Chemoradiotherapy and Immune Checkpoint Inhibitors: Case Report and Review of Literature
- Toxic Epidermal Necrolysis in Polymedicated Patient Treated With Radiotherapy