Clin Exp Vaccine Res.
2017 Jan;6(1):50-60. 10.7774/cevr.2017.6.1.50.
SH2 domain–containing adaptor protein B expressed in dendritic cells is involved in T-cell homeostasis by regulating dendritic cell–mediated Th2 immunity
- Affiliations
-
- 1Department of Biological Science, Sungkyunkwan University, Suwon, Korea. ysbae04@skku.edu
- KMID: 2366892
- DOI: http://doi.org/10.7774/cevr.2017.6.1.50
Abstract
- PURPOSE
The Src homology 2 domain-containing adaptor protein B (SHB) is widely expressed in immune cells and acts as an important regulator for hematopoietic cell function. SHB silencing induces Th2 immunity in mice. SHB is also involved in T-cell homeostasis in vivo. However, SHB has not yet been studied and addressed in association with dendritic cells (DCs).
MATERIALS AND METHODS
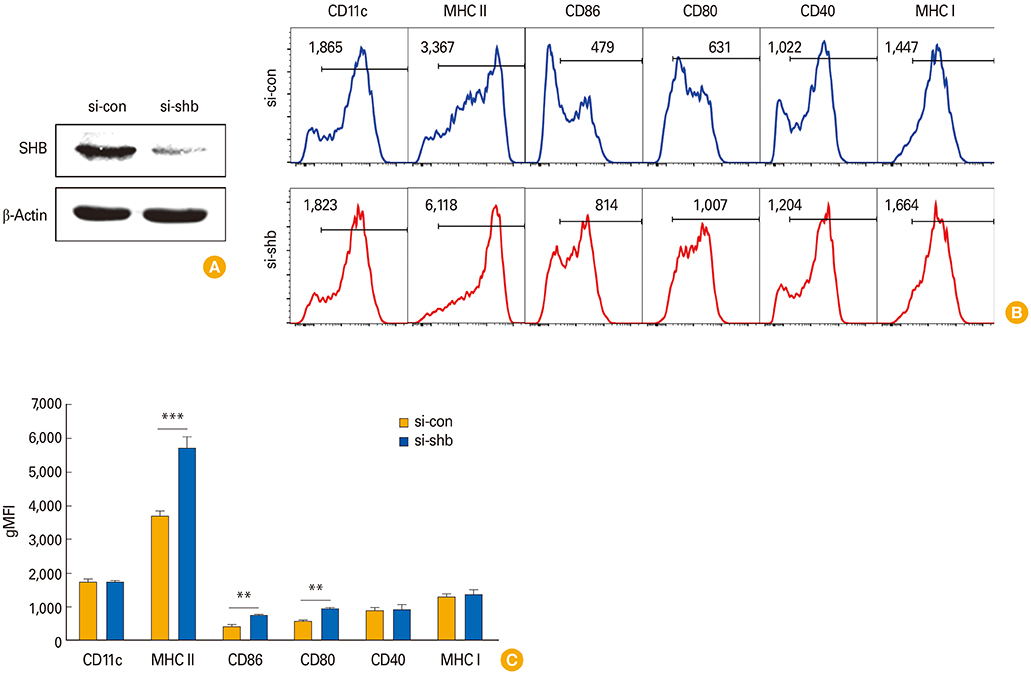
The effects of SHB expression on the immunogenicity of DCs were assessed by Shb gene silencing in mouse bone marrow-derived DCs (BMDCs). After silencing, surface phenotype, cytokine expression profile, and T-cell stimulation capacity of BMDCs were examined. We investigated the signaling pathways involved in SHB expression during BMDC development. We also examined the immunogenicity of SHB-knockdown (SHB(KD)) BMDCs in a mouse atopic dermatitis model.
RESULTS
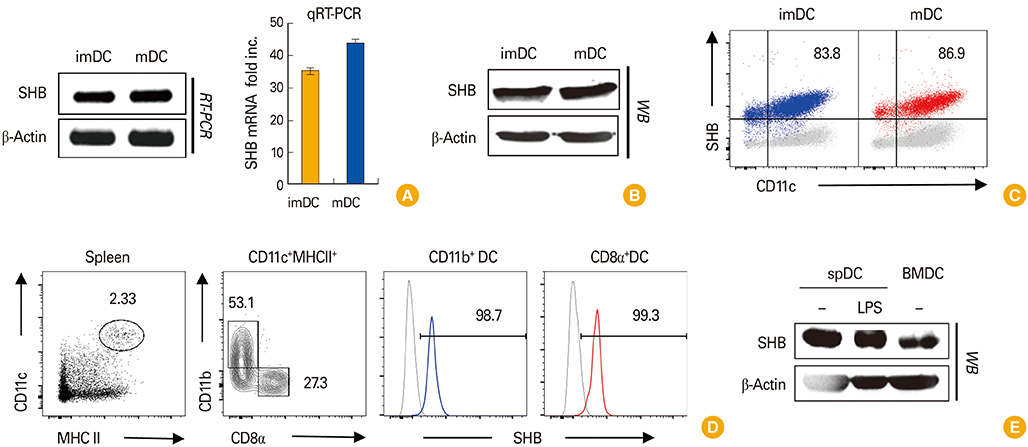
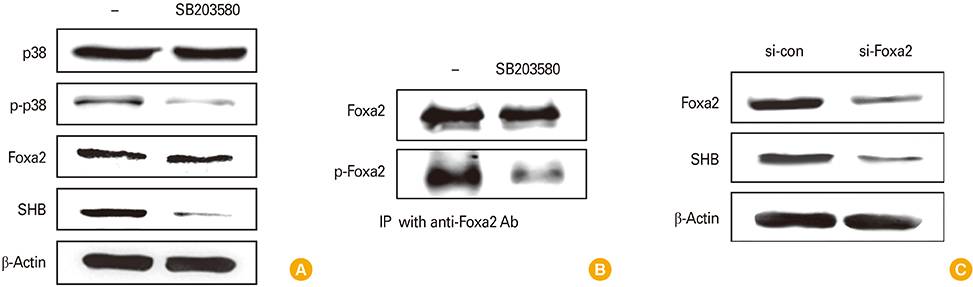
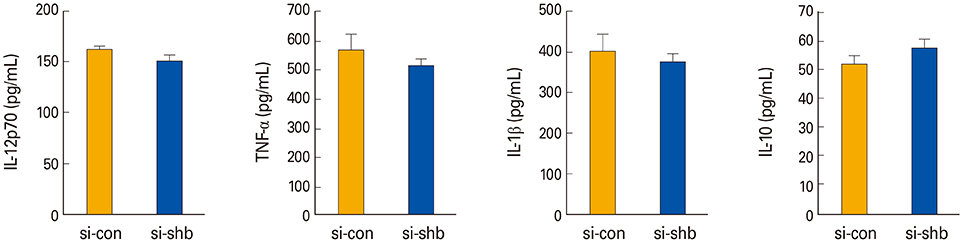
SHB was steadily expressed in mouse splenic DCs and in in vitro-generated BMDCs in both immature and mature stages. SHB expression was contingent on activation of the mitogen- activated protein kinase/Foxa2 signaling pathway during DC development. SHB(KD) increased the expression of MHC class II and costimulatory molecules without affecting the cytokine expression of BMDCs. When co-cultured with T cells, SHB(KD) in BMDCs significantly induced CD4+ T-cell proliferation and the expression of Th2 cytokines, while the regulatory T cell (Treg) population was downregulated. In mouse atopic dermatitis model, mice inoculated with SHB(KD) DCs developed more severe symptoms of atopic dermatitis compared with mice injected with control DCs.
CONCLUSION
SHB expression in DCs plays an important role in T-cell homeostasis in vivo by regulating DC-mediated Th2 polarization.
Keyword
MeSH Terms
Figure
Reference
-
1. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998; 392:245–252.
Article2. Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003; 19:641–644.3. Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005; 6:1123–1132.
Article4. Lyerly HK, Clay T, Morse MA. Optimizing dendritic cell function by genetic modification. J Natl Cancer Inst. 2000; 92:1198–1199.
Article5. Miah MA, Byeon SE, Ahmed MS, Yoon CH, Ha SJ, Bae YS. Egr2 induced during DC development acts as an intrinsic negative regulator of DC immunogenicity. Eur J Immunol. 2013; 43:2484–2496.
Article6. Ahmed MS, Byeon SE, Jeong Y, et al. Dab2, a negative regulator of DC immunogenicity, is an attractive molecular target for DC-based immunotherapy. Oncoimmunology. 2015; 4:e984550.
Article7. Holmqvist K, Cross MJ, Rolny C, et al. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem. 2004; 279:22267–22275.
Article8. Hooshmand-Rad R, Lu L, Heldin CH, Claesson-Welsh L, Welsh M. Platelet-derived growth factor-mediated signaling through the Shb adaptor protein: effects on cytoskeletal organization. Exp Cell Res. 2000; 257:245–254.
Article9. Welsh M, Songyang Z, Frantz JD, et al. Stimulation through the T cell receptor leads to interactions between SHB and several signaling proteins. Oncogene. 1998; 16:891–901.
Article10. Lindholm CK. IL-2 receptor signaling through the Shb adapter protein in T and NK cells. Biochem Biophys Res Commun. 2002; 296:929–936.
Article11. Lindholm CK, Henriksson ML, Hallberg B, Welsh M. Shb links SLP-76 and Vav with the CD3 complex in Jurkat T cells. Eur J Biochem. 2002; 269:3279–3288.
Article12. Lindholm CK, Gylfe E, Zhang W, Samelson LE, Welsh M. Requirement of the Src homology 2 domain protein Shb for T cell receptor-dependent activation of the interleukin-2 gene nuclear factor for activation of T cells element in Jurkat T cells. J Biol Chem. 1999; 274:28050–28057.
Article13. Karlsson T, Kullander K, Welsh M. The Src homology 2 domain protein Shb transmits basic fibroblast growth factor-and nerve growth factor-dependent differentiation signals in PC12 cells. Cell Growth Differ. 1998; 9:757–766.14. Cross MJ, Lu L, Magnusson P, et al. The Shb adaptor protein binds to tyrosine 766 in the FGFR-1 and regulates the Ras/MEK/MAPK pathway via FRS2 phosphorylation in endothelial cells. Mol Biol Cell. 2002; 13:2881–2893.
Article15. van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012; 119:3383–3393.
Article16. Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006; 63:2317–2328.
Article17. Wan H, Kaestner KH, Ang SL, et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004; 131:953–964.
Article18. Chen G, Wan H, Luo F, et al. Foxa2 programs Th2 cell-mediated innate immunity in the developing lung. J Immunol. 2010; 184:6133–6141.
Article19. Gustafsson K, Calounova G, Hjelm F, et al. Shb deficient mice display an augmented TH2 response in peripheral CD4+ T cells. BMC Immunol. 2011; 12:3.
Article20. Gustafsson K, Willebrand E, Welsh M. Absence of the adaptor protein Shb potentiates the T helper type 2 response in a mouse model of atopic dermatitis. Immunology. 2014; 143:33–41.
Article21. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986; 136:2348–2357.22. McKenzie AN, Culpepper JA, de Waal Malefyt R, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993; 90:3735–3739.
Article23. Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989; 245:308–310.
Article24. Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993; 362:245–248.
Article25. Macfarlane AJ, Kon OM, Smith SJ, et al. Basophils, eosinophils, and mast cells in atopic and nonatopic asthma and in late-phase allergic reactions in the lung and skin. J Allergy Clin Immunol. 2000; 105(1 Pt 1):99–107.26. Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990; 323:1033–1039.
Article27. Mudde GC, van Reijsen FC, Bruijnzeel-Koomen CA. IgE-positive Langerhans cells and Th2 allergen-specific T cells in atopic dermatitis. J Invest Dermatol. 1992; 99:103S.
Article28. Lim DS, Kim JH, Lee DS, Yoon CH, Bae YS. DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors. Cancer Immunol Immunother. 2007; 56:1817–1829.
Article29. Lim DS, Kang MS, Jeong JA, Bae YS. Semi-mature DC are immunogenic and not tolerogenic when inoculated at a high dose in collagen-induced arthritis mice. Eur J Immunol. 2009; 39:1334–1343.
Article30. Kwon HK, Lee CG, So JS, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 2010; 107:2159–2164.
Article31. Lu T, Yang C, Sun H, Lv J, Zhang F, Dong XJ. FGF4 and HGF promote differentiation of mouse bone marrow mesenchymal stem cells into hepatocytes via the MAPK pathway. Genet Mol Res. 2014; 13:415–424.
Article