J Korean Med Sci.
2017 Mar;32(3):465-474. 10.3346/jkms.2017.32.3.465.
Epidemiology and Factors Related to Clinical Severity of Acute Gastroenteritis in Hospitalized Children after the Introduction of Rotavirus Vaccination
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea. jychangmd@hanmail.net
- 2Department of Pediatrics, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Laboratory Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea.
- 5School of Biosystem and Biomedical Science, Korea University College of Health Science, Seoul, Korea.
- 6Department of Medical Statistics, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea.
- KMID: 2366643
- DOI: http://doi.org/10.3346/jkms.2017.32.3.465
Abstract
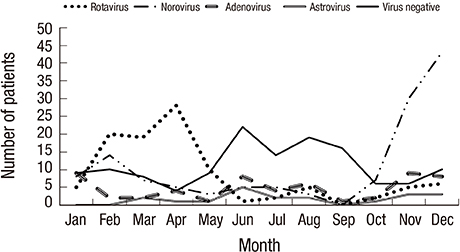
- We aimed to investigate epidemiology and host- and pathogen-related factors associated with clinical severity of acute gastroenteritis (AGE) in children after rotavirus vaccination introduction. Factors assessed included age, co-infection with more than 2 viruses, and virus-toxigenic Clostridium difficile co-detection. Fecal samples and clinical information, including modified Vesikari scores, were collected from hospitalized children with AGE. The presence of enteric viruses and bacteria, including toxigenic C. difficile, was detected by polymerase chain reaction (PCR). Among the 415 children included, virus was detected in stool of 282 (68.0%) children. Co-infection with more than 2 viruses and toxigenic C. difficile were found in 24 (8.5%) and 26 (9.2%) children with viral AGE, respectively. Norovirus (n = 130) infection, including norovirus-associated co-infection, was the most frequent infection, especially in children aged < 24 months (P < 0.001). In the severity-related analysis, age < 24 months was associated with greater diarrheal severity (P < 0.001) and modified Vesikari score (P = 0.001), after adjustment for other severity-related factors including rotavirus status. Although the age at infection with rotavirus was higher than that for other viruses (P = 0.001), rotavirus detection was the most significant risk factor for all severity parameters, including modified Vesikari score (P < 0.001). Viral co-infection and toxigenic C. difficile co-detection were not associated with any severity-related parameter. This information will be helpful in the management of childhood AGE in this era of rotavirus vaccination and availability of molecular diagnostic tests, which often lead to the simultaneous detection of multiple pathogens.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Management of Acute Gastroenteritis in Children: A Survey among Members of the Korean Society of Pediatric Gastroenterology, Hepatology, and Nutrition
Ji-Hyun Seo, Jung Ok Shim, Byung-Ho Choe, Jin Su Moon, Ki-Soo Kang, Ju-Young Chung
Pediatr Gastroenterol Hepatol Nutr. 2019;22(5):431-440. doi: 10.5223/pghn.2019.22.5.431.
Reference
-
1. Wikswo ME, Desai R, Edwards KM, Staat MA, Szilagyi PG, Weinberg GA, Curns AT, Lopman B, Vinjé J, Parashar UD, et al. Clinical profile of children with norovirus disease in rotavirus vaccine era. Emerg Infect Dis. 2013; 19:1691–1693.2. Choi UY, Lee SY, Ma SH, Jang YT, Kim JY, Kim HM, Kim JH, Kim DS, Kim YS, Kang JH. Epidemiological changes in rotavirus gastroenteritis in children under 5 years of age after the introduction of rotavirus vaccines in Korea. Eur J Pediatr. 2013; 172:947–952.3. Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Infectious Diseases. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014; 59:132–152.4. Friesema IH, de Boer RF, Duizer E, Kortbeek LM, Notermans DW, Norbruis OF, Bezemer DD, van Heerbeek H, van Andel RN, van Enk JG, et al. Etiology of acute gastroenteritis in children requiring hospitalization in the Netherlands. Eur J Clin Microbiol Infect Dis. 2012; 31:405–415.5. Gimenez-Sanchez F, Delgado-Rubio A, Martinon-Torres F, Bernaola-Iturbe E; Rotascore Research Group. Multicenter prospective study analysing the role of rotavirus on acute gastroenteritis in Spain. Acta Paediatr. 2010; 99:738–742.6. Mathew A, Rao PS, Sowmyanarayanan TV, Kang G. Severity of rotavirus gastroenteritis in an Indian population: report from a 3 year surveillance study. Vaccine. 2014; 32:Suppl 1. A45–8.7. Strand TA, Sharma PR, Gjessing HK, Ulak M, Chandyo RK, Adhikari RK, Sommerfelt H. Risk factors for extended duration of acute diarrhea in young children. PLoS One. 2012; 7:e36436.8. Shim JO, Chang JY, Shin S, Moon JS, Ko JS. Changing distribution of age, clinical severity, and genotypes of rotavirus gastroenteritis in hospitalized children after the introduction of vaccination: a single center study in Seoul between 2011 and 2014. BMC Infect Dis. 2016; 16:287.9. Albano F, Bruzzese E, Bella A, Cascio A, Titone L, Arista S, Izzi G, Virdis R, Pecco P, Principi N, et al. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr. 2007; 166:241–247.10. Valentini D, Vittucci AC, Grandin A, Tozzi AE, Russo C, Onori M, Menichella D, Bartuli A, Villani A. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis. 2013; 32:909–915.11. Matthijnssens J, Zeller M, Heylen E, De Coster S, Vercauteren J, Braeckman T, Van Herck K, Meyer N, Pirçon JY, Soriano-Gabarro M, et al. Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin Microbiol Infect. 2014; 20:O702–10.12. Bignardi GE, Staples K, Majmudar N. A case of norovirus and Clostridium difficile infection: casual or causal relationship? J Hosp Infect. 2007; 67:198–200.13. Lukkarinen H, Eerola E, Ruohola A, Vainionpää R, Jalava J, Kotila S, Ruuskanen O. Clostridium difficile ribotype 027-associated disease in children with norovirus infection. Pediatr Infect Dis J. 2009; 28:847–848.14. Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990; 22:259–267.15. Allard A, Girones R, Juto P, Wadell G. Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol. 1990; 28:2659–2667.16. Koh H, Baek SY, Shin JI, Chung KS, Jee YM. Coinfection of viral agents in Korean children with acute watery diarrhea. J Korean Med Sci. 2008; 23:937–940.17. Belliot G, Laveran H, Monroe SS. Detection and genetic differentiation of human astroviruses: phylogenetic grouping varies by coding region. Arch Virol. 1997; 142:1323–1334.18. Yang HR, Jee YM, Ko JS, Seo JK. Detection and genotyping of viruses detected in children with benign afebrile seizures associated with acute gastroenteritis. Korean J Pediatr Gastroenterol Nutr. 2009; 12:183–193.19. Higgins RR, Beniprashad M, Cardona M, Masney S, Low DE, Gubbay JB. Evaluation and verification of the Seeplex Diarrhea-V ACE assay for simultaneous detection of adenovirus, rotavirus, and norovirus genogroups I and II in clinical stool specimens. J Clin Microbiol. 2011; 49:3154–3162.20. Onori M, Coltella L, Mancinelli L, Argentieri M, Menichella D, Villani A, Grandin A, Valentini D, Raponi M, Russo C. Evaluation of a multiplex PCR assay for simultaneous detection of bacterial and viral enteropathogens in stool samples of paediatric patients. Diagn Microbiol Infect Dis. 2014; 79:149–154.21. Jin HI, Lee YM, Choi YJ, Jeong SJ. Recent viral pathogen in acute gastroenteritis: a retrospective study at a tertiary hospital for 1 year. Korean J Pediatr. 2016; 59:120–125.22. Doll MK, Gagneur A, Tapiéro B, Charest H, Gonzales M, Buckeridge DL, Quach C. Temporal changes in pediatric gastroenteritis after rotavirus vaccination in Quebec. Pediatr Infect Dis J. 2016; 35:555–560.23. Lopman BA, Steele D, Kirkwood CD, Parashar UD. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med. 2016; 13:e1001999.24. Chung JY, Huh K, Kim SW, Shin BM, Han TH, Lee JI, Song MO. Molecular epidemiology of human astrovirus infection in hospitalized children with acute gastroenteritis. Korean J Pediatr Gastroenterol Nutr. 2006; 9:139–146.25. Lee JI, Lee GC, Chung JY, Han TH, Lee YK, Kim MS, Lee CH. Detection and molecular characterization of adenoviruses in Korean children hospitalized with acute gastroenteritis. Microbiol Immunol. 2012; 56:523–528.26. Chen CJ, Wu FT, Huang YC, Chang WC, Wu HS, Wu CY, Lin JS, Huang FC, Hsiung CA. Clinical and epidemiologic features of severe viral gastroenteritis in children: a 3-year surveillance, multicentered study in Taiwan with partial rotavirus immunization. Medicine (Baltimore). 2015; 94:e1372.27. Thongprachum A, Takanashi S, Kalesaran AF, Okitsu S, Mizuguchi M, Hayakawa S, Ushijima H. Four-year study of viruses that cause diarrhea in Japanese pediatric outpatients. J Med Virol. 2015; 87:1141–1148.28. Reis TA, Assis AS, do Valle DA, Barletta VH, de Carvalho IP, Rose TL, Portes SA, Leite JP. da Rosa e Silva ML. The role of human adenoviruses type 41 in acute diarrheal disease in Minas Gerais after rotavirus vaccination. Braz J Microbiol. 2016; 47:243–250.29. Tran A, Talmud D, Lejeune B, Jovenin N, Renois F, Payan C, Leveque N, Andreoletti L. Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in northern France. J Clin Microbiol. 2010; 48:1943–1946.30. Román E, Wilhelmi I, Colomina J, Villar J, Cilleruelo ML, Nebreda V, Del Alamo M, Sánchez-Fauquier A. Acute viral gastroenteritis: proportion and clinical relevance of multiple infections in Spanish children. J Med Microbiol. 2003; 52:435–440.31. Lu L, Jia R, Zhong H, Xu M, Su L, Cao L, Dong Z, Dong N, Xu J. Molecular characterization and multiple infections of rotavirus, norovirus, sapovirus, astrovirus and adenovirus in outpatients with sporadic gastroenteritis in Shanghai, China, 2010-2011. Arch Virol. 2015; 160:1229–1238.32. Marie-Cardine A, Gourlain K, Mouterde O, Castignolles N, Hellot MF, Mallet E, Buffet-Janvresse C. Epidemiology of acute viral gastroenteritis in children hospitalized in Rouen, France. Clin Infect Dis. 2002; 34:1170–1178.33. González GG, Liprandi F, Ludert JE. Molecular epidemiology of enteric viruses in children with sporadic gastroenteritis in Valencia, Venezuela. J Med Virol. 2011; 83:1972–1982.34. Cerquetti M, Luzzi I, Caprioli A, Sebastianelli A, Mastrantonio P. Role of Clostridium difficile in childhood diarrhea. Pediatr Infect Dis J. 1995; 14:598–603.35. Mårdh PA, Helin I, Colleen I, Oberg M, Holst E. Clostridium difficile toxin in faecal specimens of healthy children and children with diarrhoea. Acta Paediatr Scand. 1982; 71:275–278.36. de Graaf H, Pai S, Burns DA, Karas JA, Enoch DA, Faust SN. Co-infection as a confounder for the role of Clostridium difficile infection in children with diarrhoea: a summary of the literature. Eur J Clin Microbiol Infect Dis. 2015; 34:1281–1287.37. Rexach CE, Tang-Feldman YJ, Cantrell MC, Cohen SH. Epidemiologic surveillance of Clostridium difficile diarrhea in a freestanding pediatric hospital and a pediatric hospital at a university medical center. Diagn Microbiol Infect Dis. 2006; 56:109–114.38. El Feghaly RE, Stauber JL, Tarr PI, Haslam DB. Viral co-infections are common and are associated with higher bacterial burden in children with Clostridium difficile infection. J Pediatr Gastroenterol Nutr. 2013; 57:813–816.39. Schutze GE, Willoughby RE; Committee on Infectious Diseases. American Academy of Pediatrics. Clostridium difficile infection in infants and children. Pediatrics. 2013; 131:196–200.40. Borali E, De Giacomo C. Clostridium difficile infection in children: a review. J Pediatr Gastroenterol Nutr. 2016; 63:e130–40.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical and Epidemiological Study of 1,165 Hospitalized Cases of Rotaviral Gastroenteritis Before and After the Introduction of Rotavirus Vaccine, 2006-2013
- The Changes in the Outbreak of Rotavirus Gastroenteritis in Children after Introduction of Rotavirus Vaccines: A Retrospective Study at a Tertiary Hospital
- Clinical ep idemiologic profile of rotavirus infections in Korea
- Changes in the Occurrence of Rotavirus Gastroenteritis before and after the Introduction of Rotavirus Vaccine among Hospitalized Pediatric Patients and Estimates of Rotavirus Vaccine Effectiveness
- Rotavirus Vaccine