Tuberc Respir Dis.
2016 Oct;79(4):207-213. 10.4046/trd.2016.79.4.207.
A Mitochondrial Perspective of Chronic Obstructive Pulmonary Disease Pathogenesis
- Affiliations
-
- 1Section of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA. min-jong.kang@yale.edu
- 2Department of Pathology, Yale University School of Medicine, New Haven, CT, USA.
- 3Department of Genetics, Yale University School of Medicine, New Haven, CT, USA.
- KMID: 2365312
- DOI: http://doi.org/10.4046/trd.2016.79.4.207
Abstract
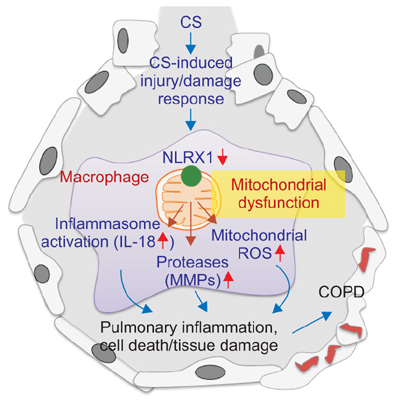
- Chronic obstructive pulmonary disease (COPD) encompasses several clinical syndromes, most notably emphysema and chronic bronchitis. Most of the current treatments fail to attenuate severity and progression of the disease, thereby requiring better mechanistic understandings of pathogenesis to develop disease-modifying therapeutics. A number of theories on COPD pathogenesis have been promulgated wherein an increase in protease burden from chronic inflammation, exaggerated production of reactive oxygen species and the resulting oxidant injury, or superfluous cell death responses caused by enhanced cellular injury/damage were proposed as the culprit. These hypotheses are not mutually exclusive and together likely represent the multifaceted biological processes involved in COPD pathogenesis. Recent studies demonstrate that mitochondria are involved in innate immune signaling that plays important roles in cigarette smoke-induced inflammasome activation, pulmonary inflammation and tissue remodeling responses. These responses are reviewed herein and synthesized into a view of COPD pathogenesis whereby mitochondria play a central role.
MeSH Terms
Figure
Reference
-
1. Senior RM, Shapiro SD. Chronic obstructive pulmonary disease: epidemiology, pathophysiology, and pathogenesis. In : Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman's pulmonary diseases and disorders. 3rd ed. New York: McGraw-Hill, Inc.;1998. p. 659–681.2. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009; 4:435–459.3. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global burden of disease and risk factors. Washington DC: The International Bank for Reconstruction and Development/The World Bank Group;2006.4. Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012; 148:1145–1159.5. Eriksson S. Pulmonary emphysema and alpha1-antitrypsin deficiency. Acta Med Scand. 1964; 175:197–205.6. MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005; 2:50–60.7. Larsson K. Aspects on pathophysiological mechanisms in COPD. J Intern Med. 2007; 262:311–340.8. Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011; 6:413–421.9. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007; 176:532–555.10. Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974; 291:755–758.11. Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smokinginduced emphysema. Nat Med. 2007; 13:567–569.12. Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009; 360:2445–2454.13. Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011; 378:1015–1026.14. Kang MJ, Choi JM, Kim BH, Lee CM, Cho WK, Choe G, et al. IL-18 induces emphysema and airway and vascular remodeling via IFN-gamma, IL-17A, and IL-13. Am J Respir Crit Care Med. 2012; 185:1205–1217.15. Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol. 2007; 178:1948–1959.16. Dima E, Koltsida O, Katsaounou P, Vakali S, Koutsoukou A, Koulouris NG, et al. Implication of interleukin (IL)-18 in the pathogenesis of chronic obstructive pulmonary disease (COPD). Cytokine. 2015; 74:313–317.17. Fischer BM, Voynow JA, Ghio AJ. COPD: balancing oxidants and antioxidants. Int J Chron Obstruct Pulmon Dis. 2015; 10:261–276.18. Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art: cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc. 2006; 3:503–510.19. Yoshida T, Tuder RM. Pathobiology of cigarette smokeinduced chronic obstructive pulmonary disease. Physiol Rev. 2007; 87:1047–1082.20. Imai K, Mercer BA, Schulman LL, Sonett JR, D'Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J. 2005; 25:250–258.21. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013; 153:1194–1217.22. Kirkwood TB. Understanding the odd science of aging. Cell. 2005; 120:437–447.23. Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc. 2009; 6:570–572.24. Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging. 2013; 8:1489–1496.25. Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015; 70:482–489.26. Holloway RA, Donnelly LE. Immunopathogenesis of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2013; 19:95–102.27. Scheffler IE. Mitochondria. 2nd ed. Hoboken: Wiley-Liss;2007.28. West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011; 11:389–402.29. Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015; 42:406–417.30. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005; 120:483–495.31. Cloonan SM, Choi AM. Mitochondria in lung disease. J Clin Invest. 2016; 126:809–820.32. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012; 481:278–286.33. Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011; 29:707–735.34. Schroder K, Tschopp J. The inflammasomes. Cell. 2010; 140:821–832.35. Yoon CM, Nam M, Oh YM, Dela Cruz CS, Kang MJ. Mitochondrial regulation of inflammasome activation in chronic obstructive pulmonary disease. J Innate Immun. 2016; 8:121–128.36. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417:1–13.37. Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012; 13:780–788.38. Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015; 163:560–569.39. Murphy MP. Modulating mitochondrial intracellular location as a redox signal. Sci Signal. 2012; 5:pe39.40. Bhola PD, Letai A. Mitochondria-judges and executioners of cell death sentences. Mol Cell. 2016; 61:695–704.41. Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nat Cell Biol. 2014; 16:728–736.42. Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005; 122:669–682.43. Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, et al. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008; 118:2771–2784.44. Kang MJ, Yoon CM, Kim BH, Lee CM, Zhou Y, Sauler M, et al. Suppression of NLRX1 in chronic obstructive pulmonary disease. J Clin Invest. 2015; 125:2458–2462.45. Chang CH, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O'Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013; 153:1239–1251.46. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013; 38:225–236.47. Arnoult D, Soares F, Tattoli I, Castanier C, Philpott DJ, Girardin SE. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J Cell Sci. 2009; 122(Pt 17):3161–3168.48. Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011; 12:901–910.49. Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008; 451:573–577.50. Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011; 34:854–865.51. Lei Y, Wen H, Yu Y, Taxman DJ, Zhang L, Widman DG, et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012; 36:933–946.52. Koblansky AA, Truax AD, Liu R, Montgomery SA, Ding S, Wilson JE, et al. The innate immune receptor NLRX1 functions as a tumor suppressor by reducing colon tumorigenesis and key tumor-promoting signals. Cell Rep. 2016; 14:2562–2575.53. Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014; 5:435.54. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013; 14:986–995.55. Byrne AJ, Mathie SA, Gregory LG, Lloyd CM. Pulmonary macrophages: key players in the innate defence of the airways. Thorax. 2015; 70:1189–1196.56. Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989; 170:499–509.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathogenesis of Chronic Obstructive Pulmonary Disease: A Role for Apoptosis
- Chronic Obstructive Pulmonary Disease: Respiratory Review of 2014
- Cor Pulmonale with Particular Reference to Chronic Obstructive Pulmonary Disease and Pulmonary Tuberculosis
- Pathogenesis and pathophysiology of COPD
- Recent Advances in Molecular Basis of Lung Aging and Its Associated Diseases