J Korean Soc Surg Hand.
2016 Dec;21(4):189-197. 10.12790/jkssh.2016.21.4.189.
The Effect of Platelet Rich Plasma Dosage on the Tendon Healing in Rabbits
- Affiliations
-
- 1Aesthetic, Plastic and Reconstructive Surgery Center, Good Moonhwa Hospital, Busan, Korea. Limon0910@gmail.com
- KMID: 2364209
- DOI: http://doi.org/10.12790/jkssh.2016.21.4.189
Abstract
- PURPOSE
Autologous platelet rich plasma (PRP) has been known to enhance tendon healing and improve tensile strength after tendon injury. This study investigated the dosage of PRP to increase the tensile strength.
METHODS
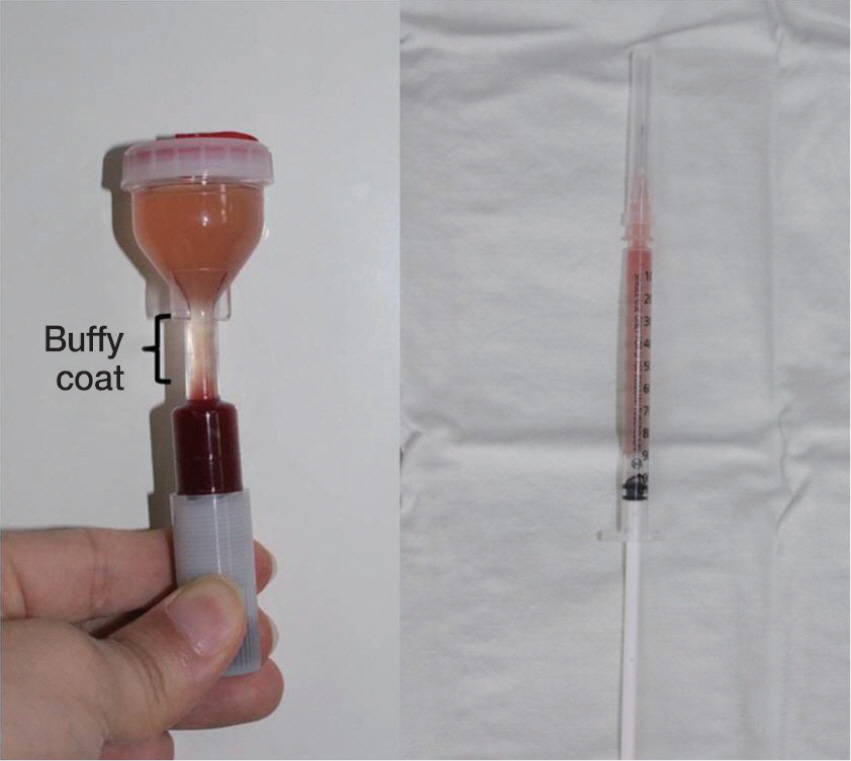
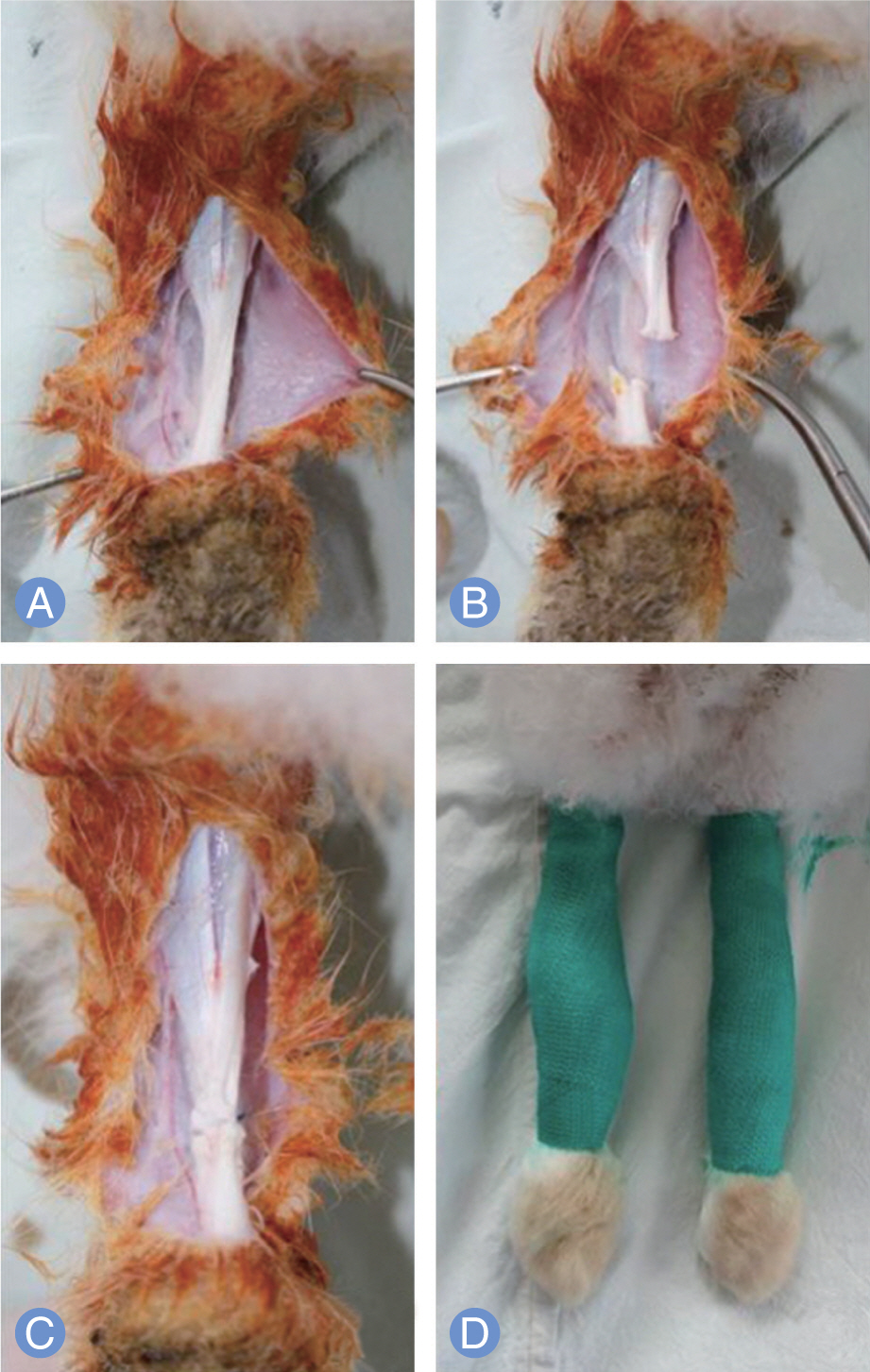
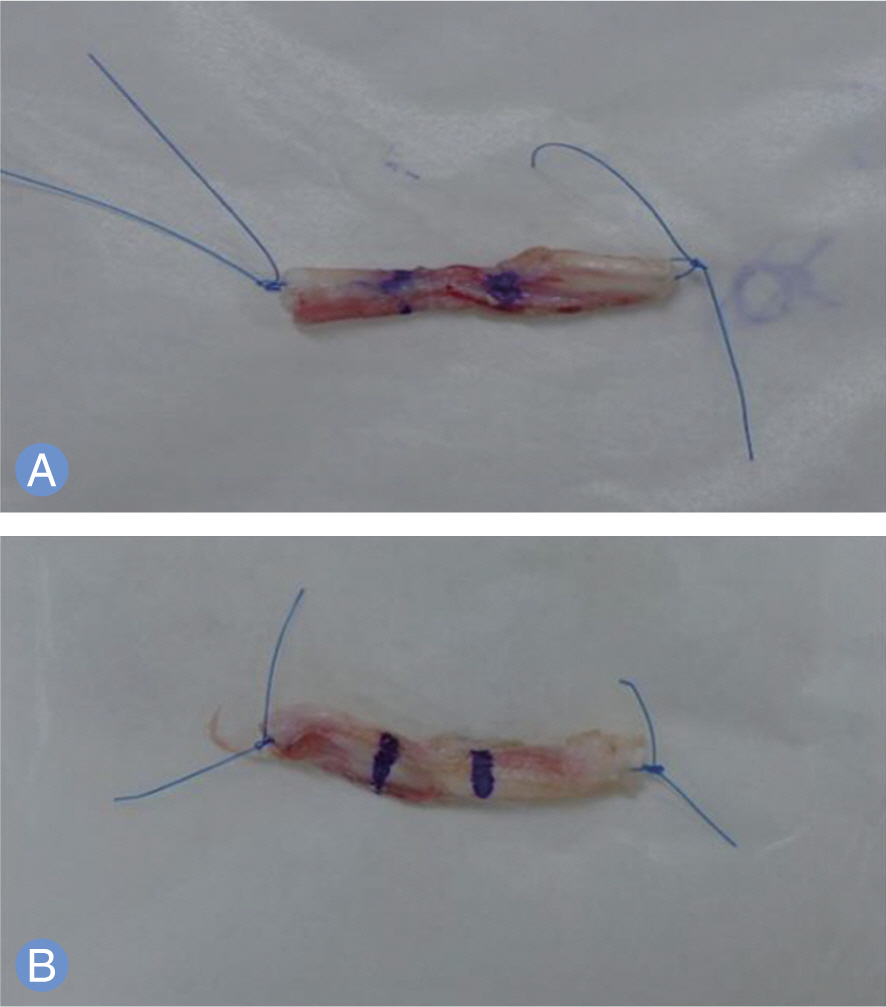
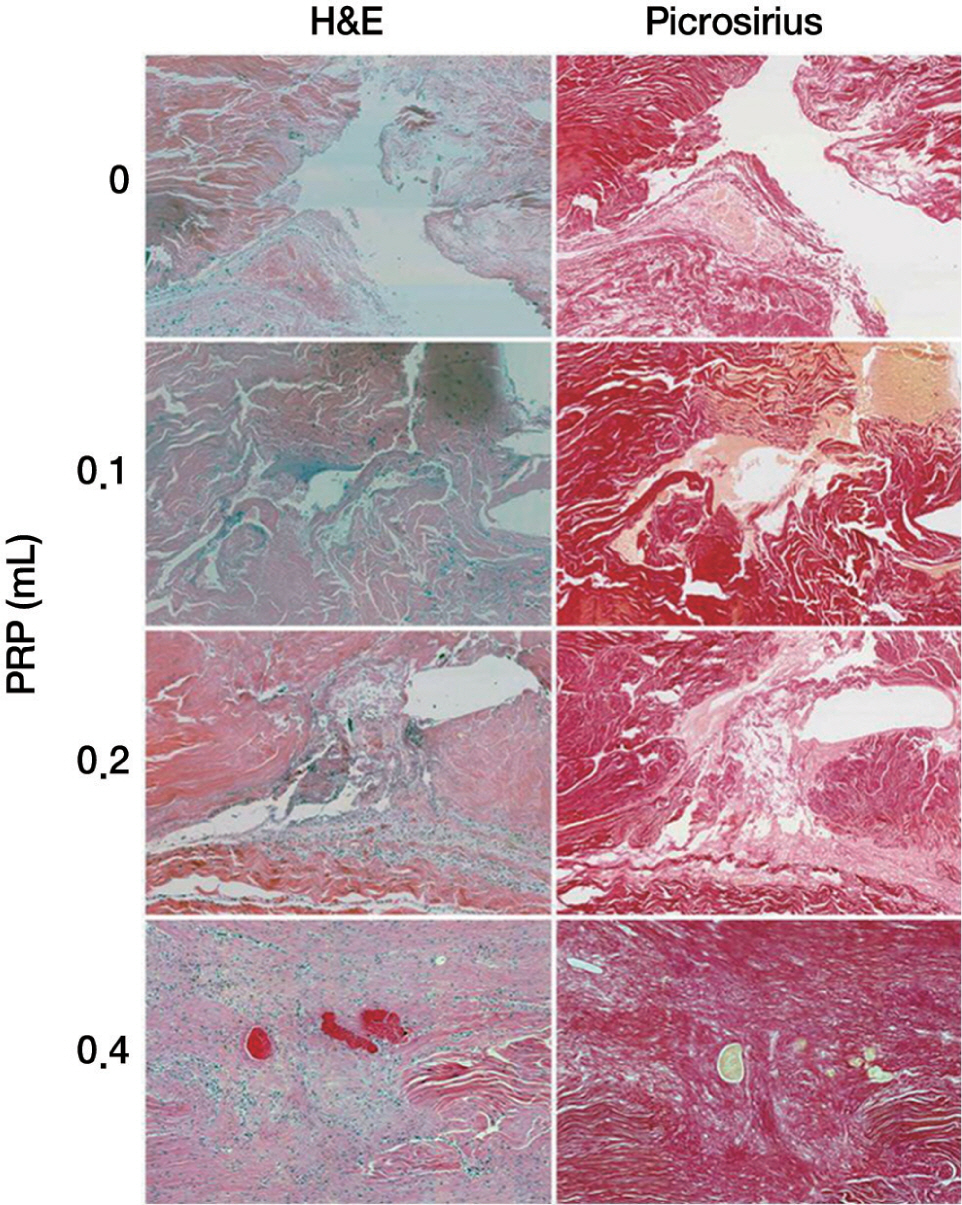
PRP was harvested from peripheral bloods of the rabbits. Direct injury model was adopted using 60 achilles tendons in 30 rabbits. The autologous PRP was infiltrated into the Achilles tendon repair site of four groups (control, 0.1, 0.2, 0.4 mL) with different dosages. Tendons were harvested at 2, 4 and 8 weeks and subjected to measuring mechanical tensile strength and dosage of collagen content.
RESULTS
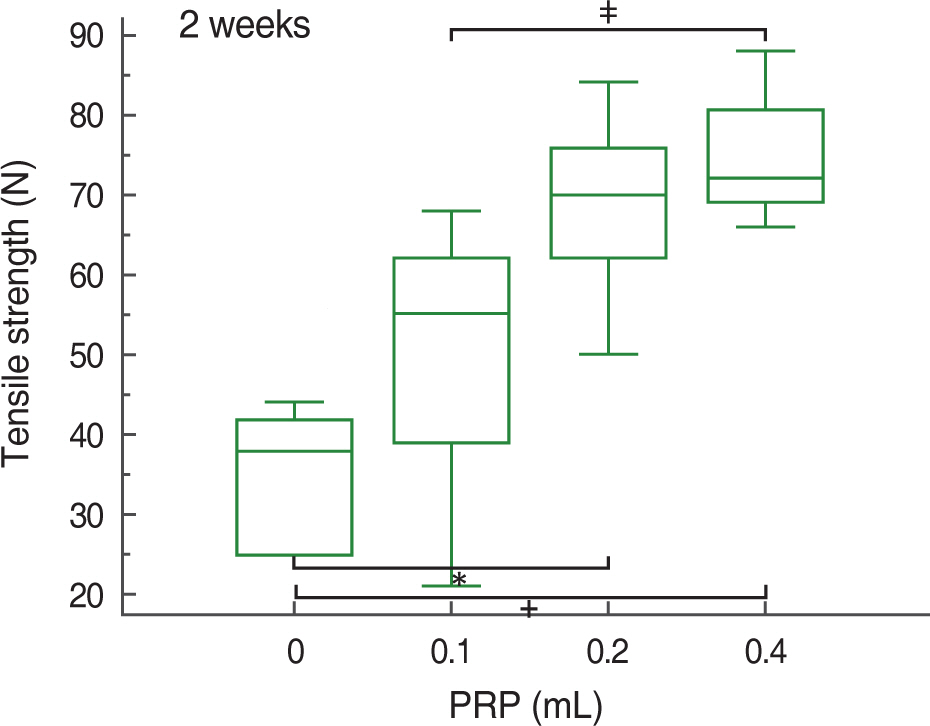
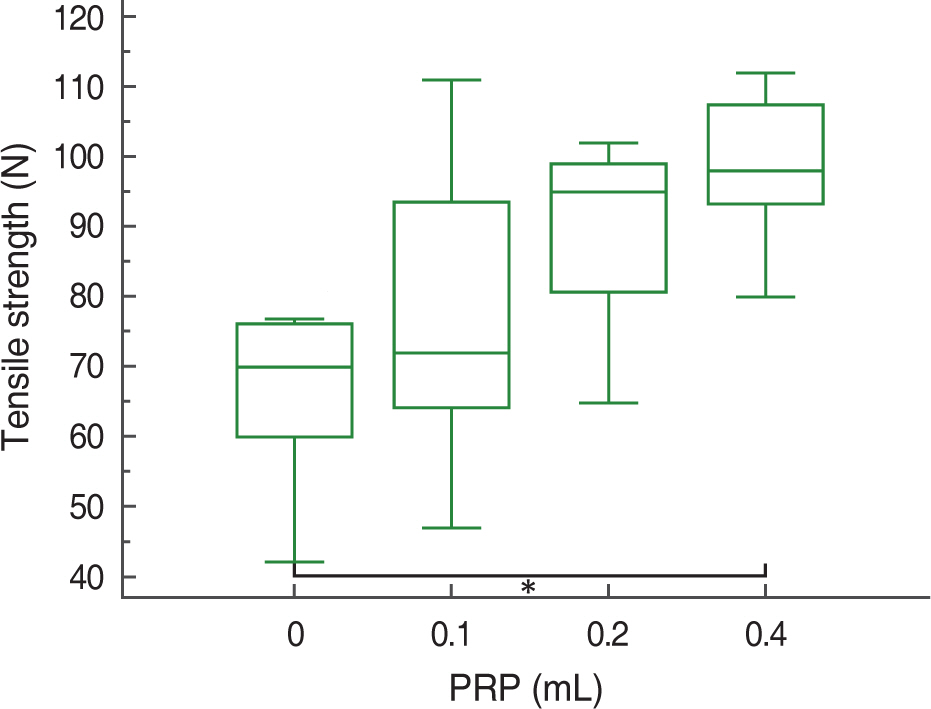
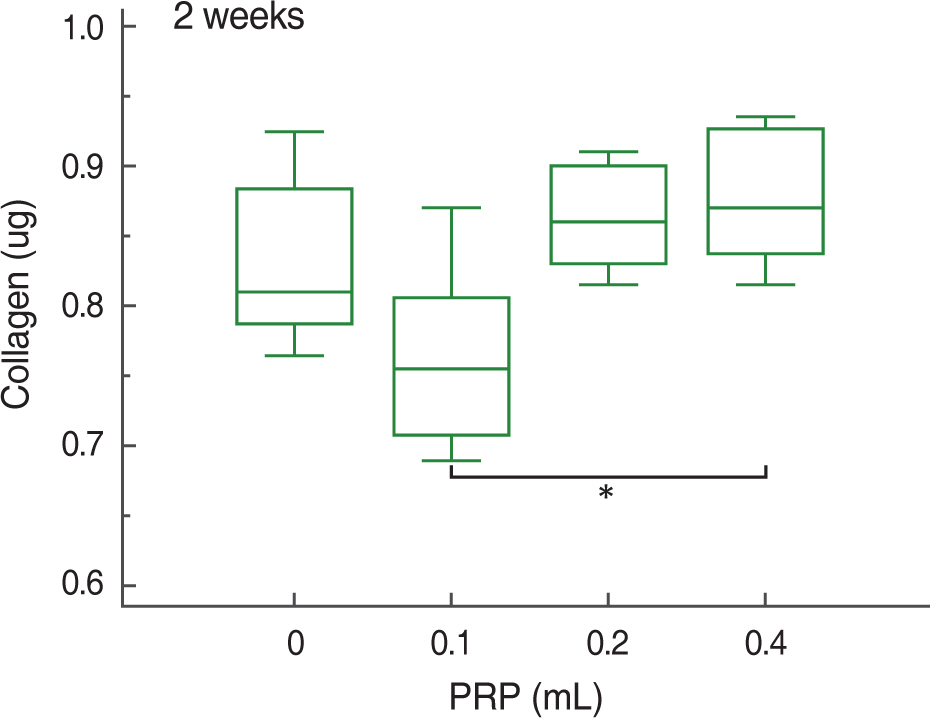
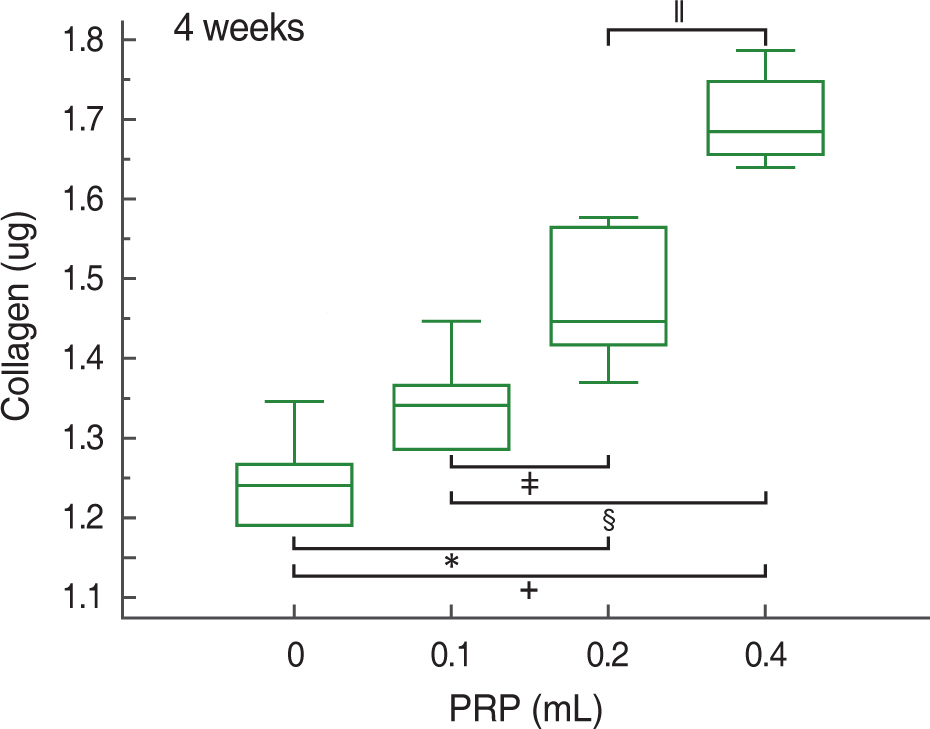
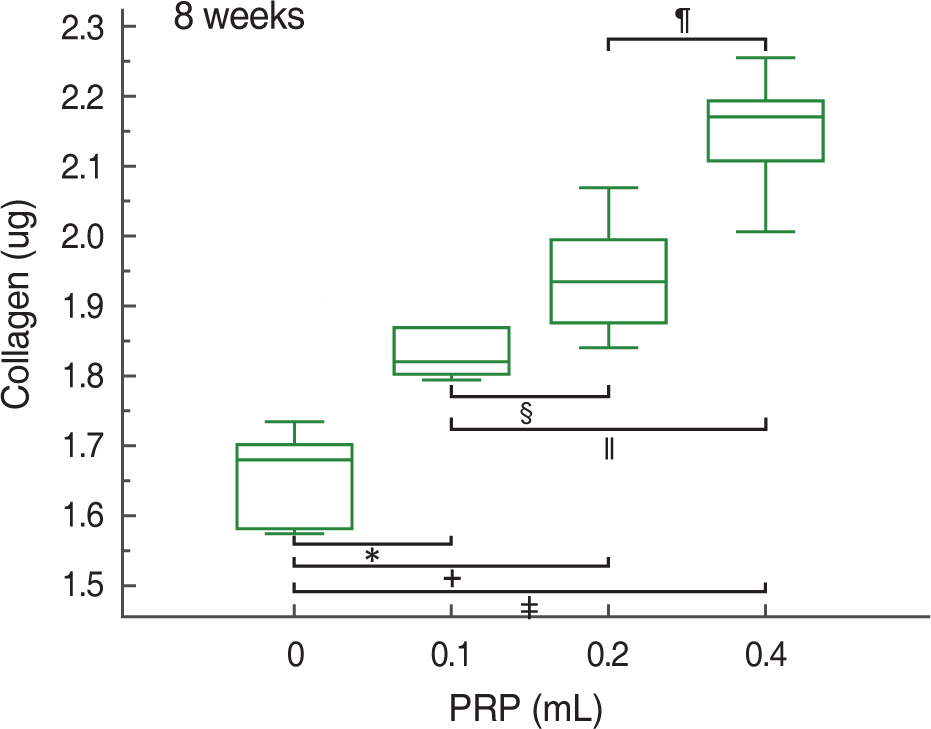
At 2, 4, and 8 weeks, PRP administration following experimental achilles tendon repair resulted in an overall higher average tensile strength and collagen content compared to these of the control. Also, the lengthen the time, tensile strength and collagen content was increased.
CONCLUSION
Autologous PRP enhanced tendon healing in rabbits. Within the PRP dosage setted by the author, more dosage of the infiltrated PRP increases the strength of the tendon and the dosage of collagen content. Further studies will be essential to determine the optimal dosage of PRP in clinical practice.
Keyword
MeSH Terms
Figure
Reference
-
1. Yao J, Woon CY, Behn A, et al. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg Am. 2012; 37:1639–45.
Article2. Kurtz CA, Loebig TG, Anderson DD, DeMeo PJ, Campbell PG. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med. 1999; 27:363–9.3. Chan BP, Fu S, Qin L, Lee K, Rolf CG, Chan K. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand. 2000; 71:513–8.
Article4. Suwalski A, Dabboue H, Delalande A, et al. Accelerated Achilles tendon healing by PDGF gene delivery with mesoporous silica nanoparticles. Biomaterials. 2010; 31:5237–45.
Article5. Liu CF, Aschbacher-Smith L, Barthelery NJ, Dyment N, Butler D, Wylie C. What we should know before using tissue engineering techniques to repair injured tendons: a developmental biology perspective. Tissue Eng Part B Rev. 2011; 17:165–76.
Article6. Blanton MW, Hadad I, Johnstone BH, et al. Adipose stromal cells and platelet-rich plasma therapies synergistically increase revascularization during wound healing. Plast Reconstr Surg. 2009; 123:56s–64s.
Article7. Kim HY, Park JH, Han YS, Kim H. The effect of platelet-rich plasma on flap survival in random extension of an axial pattern flap in rabbits. Plast Reconstr Surg. 2013; 132:85–92.
Article8. Kim HJ, Nam HW, Hur CY, et al. The effect of platelet rich plasma from bone marrow aspirate with added bone morphogenetic protein-2 on the Achilles tendon-bone junction in rabbits. Clin Orthop Surg. 2011; 3:325–31.
Article9. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012; 40:2037–44.
Article10. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014; 134:1271–7.
Article11. Guevara-Alvarez A, Schmitt A, Russell RP, Imhoff AB, Buchmann S. Growth factor delivery vehicles for tendon injuries: Mesenchymal stem cells and Platelet Rich Plasma. Muscles Ligaments Tendons J. 2014; 4:378–85.
Article12. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008; 36:1171–8.13. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006; 17:212–9.
Article14. Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006; 6:181–90.15. Shin KH, Lee H, Kang S, et al. Effect of leukocyte-rich and platelet-rich plasma on healing of a horizontal medial meniscus tear in a rabbit model. Biomed Res Int. 2015; 2015:179756.
Article16. Sen B, Guler S, Cecen B, et al. The effect of autologous platelet rich plasma in the treatment of achilles tendon ruptures: an experimental study on rabbits. Balkan Med J. 2016; 33:94–101.
Article17. Fukawa T, Yamaguchi S, Watanabe A, et al. quantitative assessment of tendon healing by using MR T2 mapping in a rabbit achilles tendon transection model treated with platelet-rich plasma. Radiology. 2015; 276:748–55.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of platelet-rich plasma in Achilles tendon allograft in rabbits
- Effect of Platelet-rich Plasma on Burn Wounds according to Time of Application: An Experimental Study on Rats
- The Effect of Platelet-Rich Plasma (PRP) on the Healing of Allograft for the Treatment of Segmental Bone Defect of the Ulna in Rabbits
- The Effect of Platelet Rich Plasma from Bone Marrow Aspirate with Added Bone Morphogenetic Protein-2 on the Achilles Tendon-Bone Junction in Rabbits
- Platelet-rich plasma: a healing virtuoso