Clin Exp Otorhinolaryngol.
2016 Dec;9(4):352-357. 10.21053/ceo.2015.01648.
Age-Related Changes in Antioxidative Enzyme Capacity in Tongue of Fischer 344 Rats
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Gachon University Gil Hospital, Incheon, Korea. hndyk@gilhospital.com
- 2Medical Research Institute, Gachon University Gil Hospital, Incheon, Korea.
- KMID: 2360767
- DOI: http://doi.org/10.21053/ceo.2015.01648
Abstract
OBJECTIVES
Antioxidative enzyme efficiency changes in some organs with age. However, no study has been conducted on age-related antioxidant enzyme changes in tongue. In the present study, the authors investigated the activities of four antioxidative enzymes and their protein expressions in the tongues of young and old Fischer 344 rats.
METHODS
Age-dependent changes in the enzyme activities of total superoxide dismutase (SOD), Mn-SOD, Cu/Zn-SOD, catalase (CAT), and glutathione peroxidase (GPx) were determined using chemical kits, and the protein expressions levels of these enzymes by Western blotting. The study was conducted using rats aged 7 months (the young group, n=8) and 22 months (the old group, n=8).
RESULTS
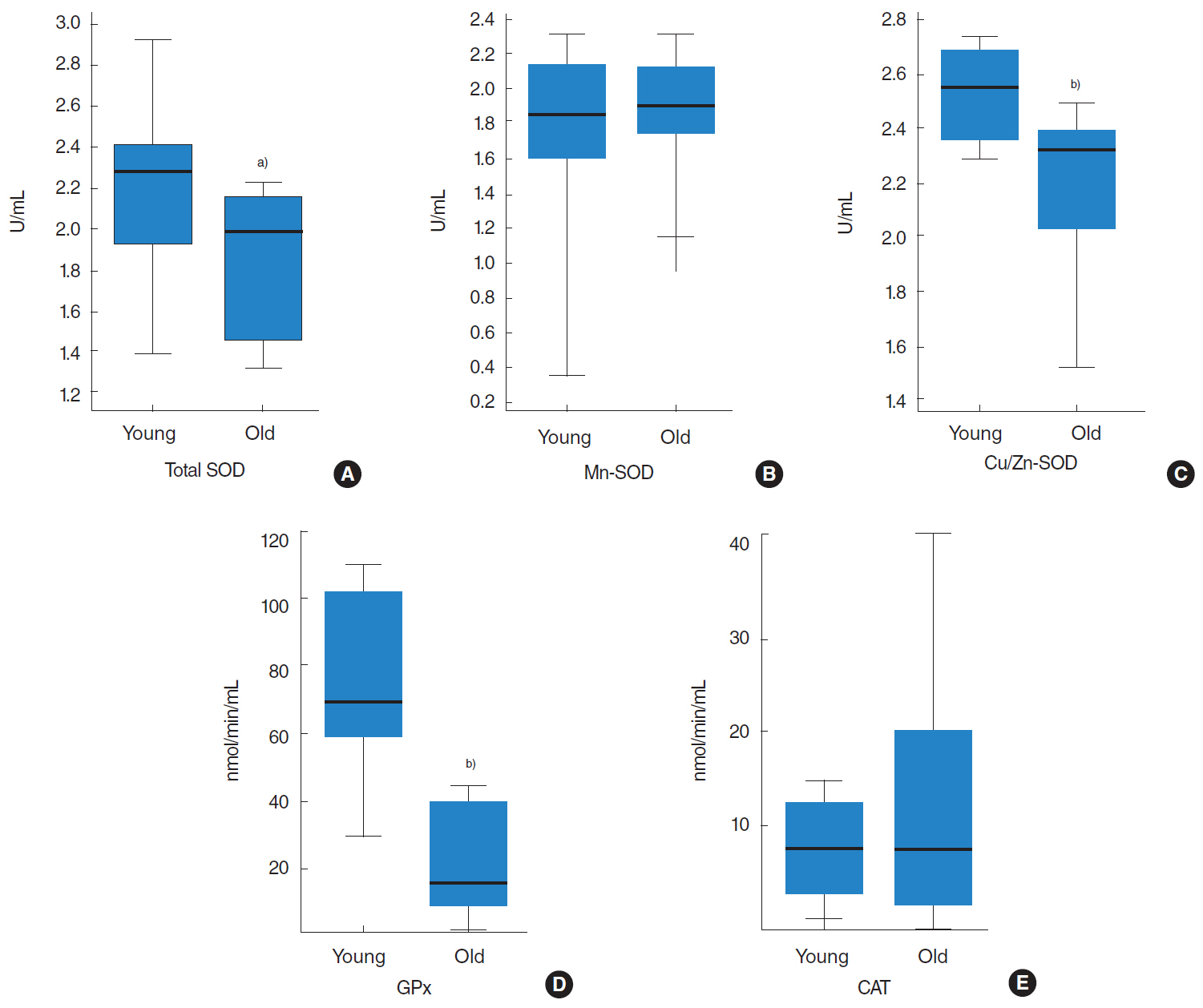
Total SOD, Cu/Zn-SOD, and GPx activities in the tongues of old rats were lower than in young rats, and similarly, corresponding protein expressions were downregulated in old rats. On the other hand, although the protein expressions of Mn-SOD and CAT were lower in old rats, their enzyme activities were not.
CONCLUSION
The results of this study provide a possible mechanism for the tongue aging process, as in old Fischer 344 rats the antioxidant defense system was diminished with respect to enzyme activity levels and protein abundances.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Age-Related Changes in Nuclear Factor Erythroid 2-Related Factor 2 and Reactive Oxygen Species and Mitochondrial Structure in the Tongues of Fischer 344 Rats
Min-Kwan Baek, Hyon Lee, Kyung-Ok Kim, Hyun-Jin Kwon, Myung-Hee Chung, Hyoung-Min Park, Joo-Hyun Woo, Dong-Young Kim
Clin Exp Otorhinolaryngol. 2017;10(4):357-362. doi: 10.21053/ceo.2016.01095.
Reference
-
1. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956; Jul. 11(3):298–300.
Article2. Cutler RG. Human longevity and aging: possible role of reactive oxygen species. Ann N Y Acad Sci. 1991; Jul. 621:1–28.
Article3. Wong YT, Ruan R, Tay FE. Relationship between levels of oxidative DNA damage, lipid peroxidation and mitochondrial membrane potential in young and old F344 rats. Free Radic Res. 2006; Apr. 40(4):393–402.
Article4. Feuers RJ, Weindruch R, Hart RW. Caloric restriction, aging, and antioxidant enzymes. Mutat Res. 1993; Dec. 295(4-6):191–200.
Article5. Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999; Dec. 372(2):399–407.
Article6. Jung K, Henke W. Developmental changes of antioxidant enzymes in kidney and liver from rats. Free Radic Biol Med. 1996. 20(4):613.
Article7. Dogru-Abbasoglu S, Tamer-Toptani S, Ugurnal B, Kocak-Toker N, Aykac-Toker G, Uysal M. Lipid peroxidation and antioxidant enzymes in livers and brains of aged rats. Mech Ageing Dev. 1997; Nov. 98(2):177–80.8. Gomi F, Matsuo M. Effects of aging and food restriction on the antioxidant enzyme activity of rat livers. J Gerontol A Biol Sci Med Sci. 1998; May. 53(3):B161–7.
Article9. De AK, Darad R. Age-associated changes in antioxidants and antioxidative enzymes in rats. Mech Ageing Dev. 1991; Jun. 59(1-2):123–8.
Article10. Rikans LE, Hornbrook KR. Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta. 1997; Dec. 1362(2-3):116–27.
Article11. Ceballos-Picot I, Nicole A, Clement M, Bourre JM, Sinet PM. Age-related changes in antioxidant enzymes and lipid peroxidation in brains of control and transgenic mice overexpressing copper-zinc superoxide dismutase. Mutat Res. 1992; Sep. 275(3-6):281–93.
Article12. Vina J, Borras C, Gomez-Cabrera MC, Orr WC. Part of the series: from dietary antioxidants to regulators in cellular signalling and gene expression: role of reactive oxygen species and (phyto)oestrogens in the modulation of adaptive response to stress. Free Radic Res. 2006; Feb. 40(2):111–9.13. Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood). 2002; Oct. 227(9):671–82.
Article14. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; Jan. 82(1):47–95.
Article15. Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A. Oxidative stress and reactive oxygen species. Contrib Nephrol. 2005; 149:240–60.
Article16. Perno M. Burning mouth syndrome. J Dent Hyg. 2001; Summer. 75(3):245–52.17. Soudry E, Preis M, Hod R, Hamzany Y, Hadar T, Bahar G, et al. Squamous cell carcinoma of the oral tongue in patients over 75 years old. Aging Clin Exp Res. 2011; Jun. 23(3):231–5.
Article18. Pinzon Tofino ME, Gaitan Cepeda LA. Aging and the oral cavity. Pract Odontol. 1989; Mar. 10(3):33–6.19. Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005; Jun. 21(6):775–7.
Article20. Sohal RS, Arnold LA, Sohal BH. Age-related changes in antioxidant enzymes and prooxidant generation in tissues of the rat with special reference to parameters in two insect species. Free Radic Biol Med. 1990; 9(6):495–500.
Article21. Gunduz F, Senturk UK, Kuru O, Aktekin B, Aktekin MR. The effect of one year’s swimming exercise on oxidant stress and antioxidant capacity in aged rats. Physiol Res. 2004; 53(2):171–6.22. Boldyrev AA, Yuneva MO, Sorokina EV, Kramarenko GG, Fedorova TN, Konovalova GG, et al. Antioxidant systems in tissues of senescence accelerated mice. Biochemistry (Mosc). 2001; Oct. 66(10):1157–63.23. Baird MB, Samis HV Jr. Regulation of catalase activity in mice of different ages. Gerontologia. 1971; 17(2):105–15.
Article24. Siqueira IR, Fochesatto C, de Andrade A, Santos M, Hagen M, Bello-Klein A, et al. Total antioxidant capacity is impaired in different structures from aged rat brain. Int J Dev Neurosci. 2005; Dec. 23(8):663–71.
Article25. Ehrenbrink G, Hakenhaar FS, Salomon TB, Petrucci AP, Sandri MR, Benfato MS. Antioxidant enzymes activities and protein damage in rat brain of both sexes. Exp Gerontol. 2006; Apr. 41(4):368–71.
Article26. Hazelton GA, Lang CA. Glutathione peroxidase and reductase activities in the aging mouse. Mech Ageing Dev. 1985; Jan. 29(1):71–81.
Article27. Nilakantan V, Zhou X, Hilton G, Roza AM, Adams MB, Johnson CP, et al. Hierarchical change in antioxidant enzyme gene expression and activity in acute cardiac rejection: role of inducible nitric oxide synthase. Mol Cell Biochem. 2005; Feb. 270(1-2):39–47.
Article28. Kumaran S, Savitha S, Anusuya Devi M, Panneerselvam C. L-carnitine and DL-alpha-lipoic acid reverse the age-related deficit in glutathione redox state in skeletal muscle and heart tissues. Mech Ageing Dev. 2004; Jul. 125(7):507–12.29. Vinokur V, Grinberg L, Berenshtein E, Gross M, Moskovitz J, Reznick AZ, et al. Methionine-centered redox cycle in organs of the aero-digestive tract of young and old rats. Biogerontology. 2009; Feb. 10(1):43–52.
Article30. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; Jul. 70(1):158–69.31. Rao G, Xia E, Richardson A. Effect of age on the expression of antioxidant enzymes in male Fischer F344 rats. Mech Ageing Dev. 1990; Mar. 53(1):49–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Age-Related Changes in Nuclear Factor Erythroid 2-Related Factor 2 and Reactive Oxygen Species and Mitochondrial Structure in the Tongues of Fischer 344 Rats
- Effects of Different Mandarin Formulations on Antioxidative Capacity and Oxidative DNA Damage in Fifteen-Month Aged Rats
- Effect of Different Part of Mandarin Intake on Antioxidative Capacity in 15-month-old Rats
- Protective effects of garlic juice against embryotoxicity of methylmercuric chloride administered to pregnant Fischer 344 rats
- Effect of Lecithin Intake on Lipid Metabolism and Antioxidative Capacity in Rats Fed High Fat Diet