J Korean Med Sci.
2016 Feb;31(2):171-177. 10.3346/jkms.2016.31.2.171.
Differentiation of Human Dental Pulp Stem Cells into Dopaminergic Neuron-like Cells in Vitro
- Affiliations
-
- 1BioMedical Research Institute, Kyungpook National University Hospital, Daegu, Korea.
- 2Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
- 3Department of Neural Development and Disease, Korea Brain Research Institute, Daegu, Korea.
- 4Department of Urology, Kyungpook National University, Daegu, Korea. uroyoo@knu.ac.kr
- KMID: 2360038
- DOI: http://doi.org/10.3346/jkms.2016.31.2.171
Abstract
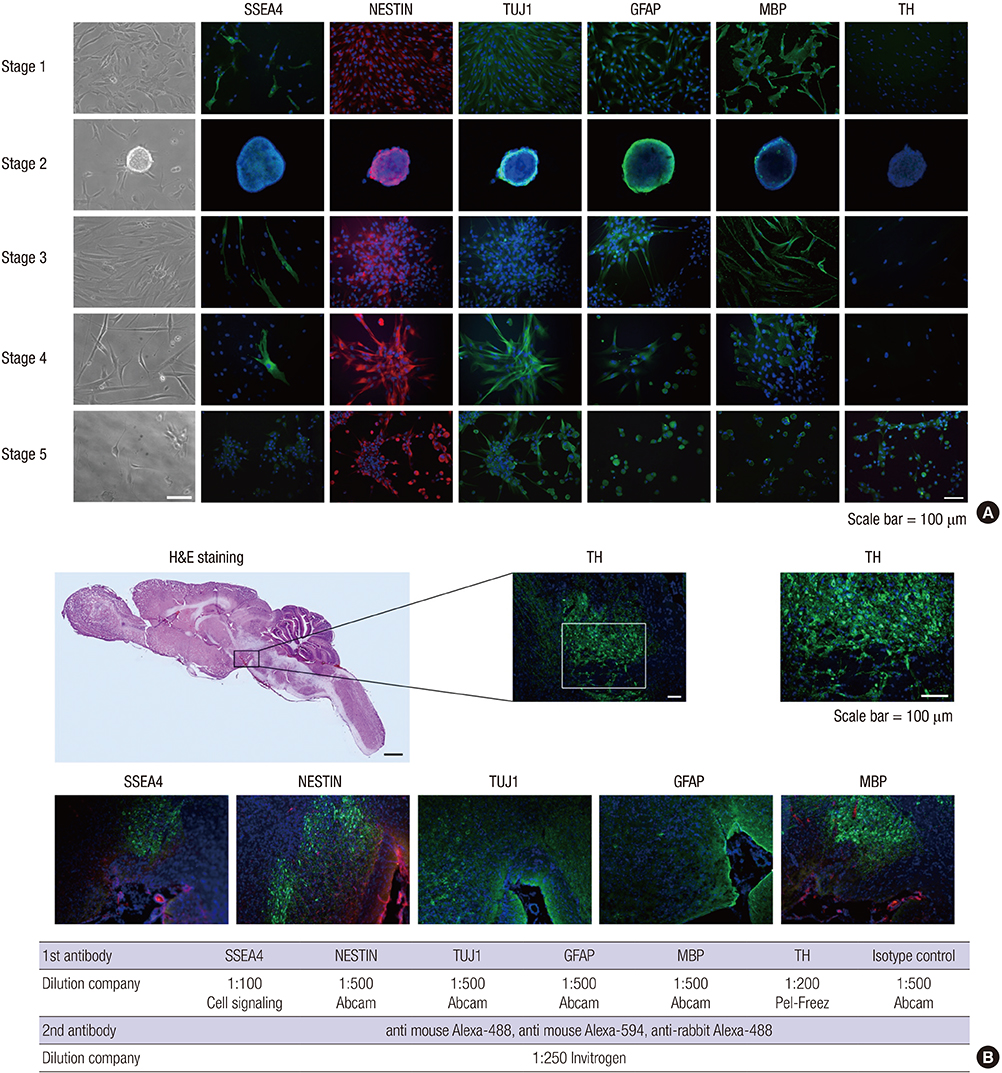
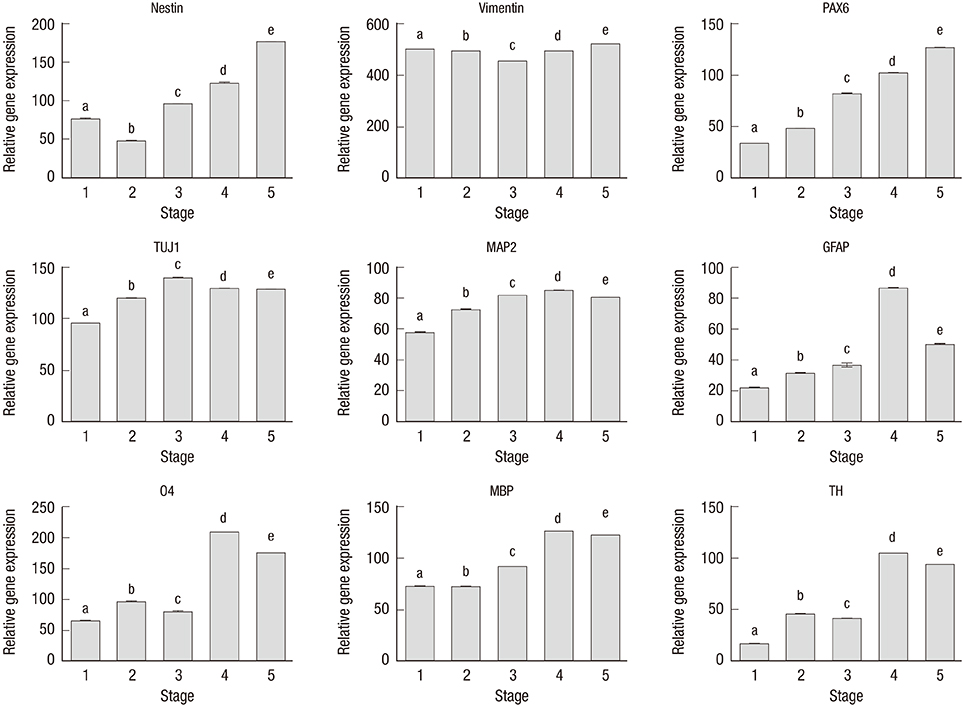
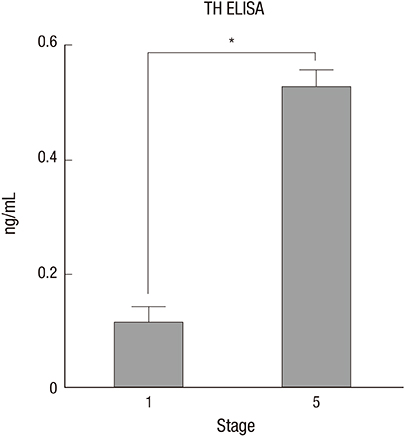
- We investigated the potential of human dental pulp stem cells (hDPSCs) to differentiate into dopaminergic neurons in vitro as an autologous stem cell source for Parkinson's disease treatment. The hDPSCs were expanded in knockout-embryonic stem cell (KO-ES) medium containing leukemia inhibitory factor (LIF) on gelatin-coated plates for 3-4 days. Then, the medium was replaced with KO-ES medium without LIF to allow the formation of the neurosphere for 4 days. The neurosphere was transferred into ITS medium, containing ITS (human insulin-transferrin-sodium) and fibronectin, to select for Nestin-positive cells for 6-8 days. The cells were then cultured in N-2 medium containing basic fibroblast growth factor (FGF), FGF-8b, sonic hedgehog-N, and ascorbic acid on poly-l-ornithine/fibronectin-coated plates to expand the Nestin-positive cells for up to 2 weeks. Finally, the cells were transferred into N-2/ascorbic acid medium to allow for their differentiation into dopaminergic neurons for 10-15 days. The differentiation stages were confirmed by morphological, immunocytochemical, flow cytometric, real-time PCR, and ELISA analyses. The expressions of mesenchymal stem cell markers were observed at the early stages. The expressions of early neuronal markers were maintained throughout the differentiation stages. The mature neural markers showed increased expression from stage 3 onwards. The percentage of cells positive for tyrosine hydroxylase was 14.49%, and the amount was 0.526 ± 0.033 ng/mL at the last stage. hDPSCs can differentiate into dopaminergic neural cells under experimental cell differentiation conditions, showing potential as an autologous cell source for the treatment of Parkinson's disease.
Keyword
MeSH Terms
-
Animals
Brain/pathology
*Cell Differentiation/drug effects
Cells, Cultured
Culture Media/chemistry/pharmacology
Dental Pulp/*cytology
Dopaminergic Neurons/*cytology/*metabolism/pathology
Enzyme-Linked Immunosorbent Assay
Glial Fibrillary Acidic Protein/genetics/metabolism
Humans
Mice
Mice, Inbred ICR
Myelin Basic Protein/genetics/metabolism
Real-Time Polymerase Chain Reaction
Stage-Specific Embryonic Antigens/genetics/metabolism
Stem Cells/*cytology/*metabolism/pathology
Tubulin/genetics/metabolism
Tyrosine 3-Monooxygenase/analysis/genetics/metabolism
Culture Media
Glial Fibrillary Acidic Protein
Myelin Basic Protein
Stage-Specific Embryonic Antigens
Tubulin
Tyrosine 3-Monooxygenase
Figure
Reference
-
1. Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003; 39:889–909.2. Mandel S, Grünblatt E, Riederer P, Gerlach M, Levites Y, Youdim MB. Neuroprotective strategies in Parkinson’s disease : an update on progress. CNS Drugs. 2003; 17:729.3. Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011; 480:547–551.4. Hayashi T, Wakao S, Kitada M, Ose T, Watabe H, Kuroda Y, Mitsunaga K, Matsuse D, Shigemoto T, Ito A, et al. Autologous mesenchymal stem cell-derived dopaminergic neurons function in parkinsonian macaques. J Clin Invest. 2013; 123:272–284.5. Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci USA. 2008; 105:12991–12996.6. de Wert G, Mummery C. Human embryonic stem cells: research, ethics and policy. Hum Reprod. 2003; 18:672–682.7. Wang Y, Chen S, Yang D, Le WD. Stem cell transplantation: a promising therapy for Parkinson’s disease. J Neuroimmune Pharmacol. 2007; 2:243–250.8. Lizier NF, Kerkis A, Gomes CM, Hebling J, Oliveira CF, Caplan AI, Kerkis I. Scaling-up of dental pulp stem cells isolated from multiple niches. PLoS One. 2012; 7:e39885.9. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000; 97:13625–13630.10. Khanna-Jain R, Vanhatupa S, Vuorinen A, Sandor GK, Suuronen R, Mannerstrom B, Miettinen S. Growth and differentiation of human dental pulp stem cells maintained in fetal bovine serum, human serum and serum-free/xeno-free culture media. J Stem Cell Res Ther. 2012; 2:1.11. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003; 100:5807–5812.12. Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006; 12:2813–2823.13. Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004; 19:2388–2398.14. Klein C, Fishell G. Neural stem cells: progenitors or panacea? Dev Neurosci. 2004; 26:82–92.15. Guo L, Yin F, Meng HQ, Ling L, Hu-He TN, Li P, Zhang CX, Yu S, Duan DS, Fan HX. Differentiation of mesenchymal stem cells into dopaminergic neuron-like cells in vitro. Biomed Environ Sci. 2005; 18:36–42.16. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000; 164:247–256.17. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370.18. Katayama M, Ishii K. 2-Mercaptoethanol-independent survival of fetal mouse brain neurons cultured in a medium of human serum. Brain Res. 1994; 656:409–412.19. Ishii K, Katayama M, Hori K, Yodoi J, Nakanishi T. Effects of 2-mercaptoethanol on survival and differentiation of fetal mouse brain neurons cultured in vitro. Neurosci Lett. 1993; 163:159–162.20. Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem. 1996; 67:1751–1760.21. Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006; 26:12089–12099.22. Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000; 28:31–40.23. Bez A, Corsini E, Curti D, Biggiogera M, Colombo A, Nicosia RF, Pagano SF, Parati EA. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003; 993:18–29.24. Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010; 19:1375–1383.25. Trzaska KA, Kuzhikandathil EV, Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007; 25:2797–2808.26. Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998; 93:755–766.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Neurogenic differentiation of human dental stem cells in vitro
- Characterization of Human Dental Pulp Cells from Supernumerary Teeth by Using Flow Cytometry Analysis
- Effects of nanoscale ridge/groovepattern arrayed surface on in vitro differentiation of multi-potent pulp cells derived from human supernumerary teeth
- Dental Pulp Stem Cells and Current in vivo Approaches to Study Dental Pulp Stem Cells in Pulp Injury and Regeneration