Korean J Hepatobiliary Pancreat Surg.
2016 Aug;20(3):110-115. 10.14701/kjhbps.2016.20.3.110.
Aggressive surgical resection for concomitant liver and lung metastasis in colorectal cancer
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. kskim88@yuhs.ac
- 2Department of Surgery, Yongin Severance Hospital, Yongin, Korea.
- 3Department of Surgery, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Thoracic and Cardiovascular Surgery, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2351297
- DOI: http://doi.org/10.14701/kjhbps.2016.20.3.110
Abstract
- BACKGROUNDS/AIMS
Aggressive surgical resection for hepatic metastasis is validated, however, concomitant liver and lung metastasis in colorectal cancer patients is equivocal.
METHODS
Clinicopathologic data from January 2008 through December 2012 were retrospectively reviewed in 234 patients with colorectal cancer with concomitant liver and lung metastasis. Clinicopathologic factors and survival data were analyzed.
RESULTS
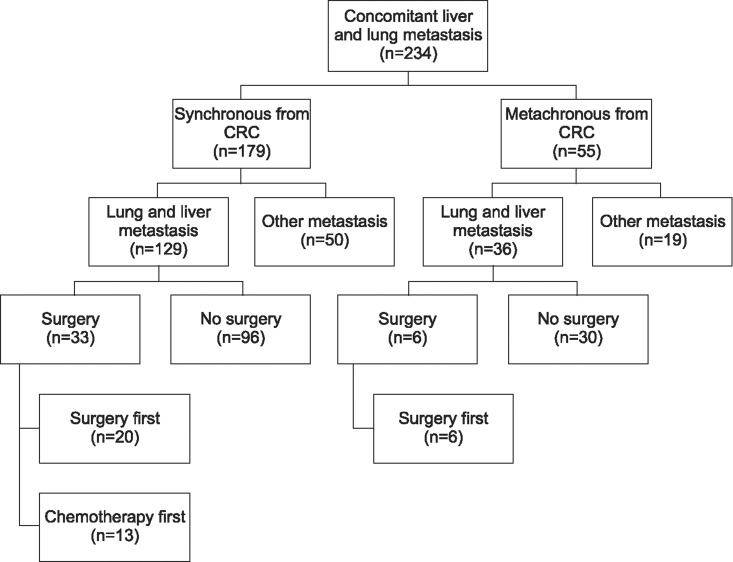
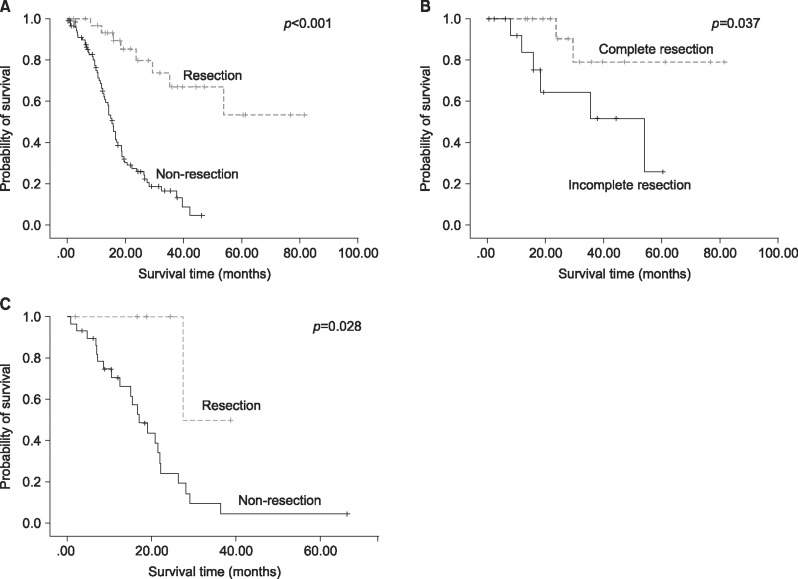
Of the 234 patients, 129 (55.1%) had synchronous concomitant liver and lung metastasis from colorectal cancer and 36 (15.4%) had metachronous metastasis. Surgical resection was performed in 33 patients (25.6%) with synchronous and 6 (16.7%) with metachronous metastasis. Surgical resection showed better overall survival in both groups (synchronous, p=0.001; metachronous, p=0.028). In the synchronous metastatic group, complete resection of both liver and lung metastatic lesions had better survival outcomes than incomplete resection of two metastatic lesions (p=0.037). The primary site of colorectal cancer and complete resection were significant prognostic factors (p=0.06 and p=0.003, respectively).
CONCLUSIONS
Surgical resection for hepatic and pulmonary metastasis in colorectal cancer can improve complete remission and survival rate in resectable cases. Colorectal cancer with concomitant liver and lung metastasis is not a poor prognostic factor or a contraindication for surgical treatments, hence, an aggressive surgical approach may be recommended in well-selected resectable cases.
Keyword
MeSH Terms
Figure
Reference
-
1. Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ Jr, Pemberton JH, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992; 174:27–32. PMID: 1729745.2. van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015; 32:457–465. PMID: 25899064.
Article3. Regnard JF, Grunenwald D, Spaggiari L, Girard P, Elias D, Ducreux M, et al. Surgical treatment of hepatic and pulmonary metastases from colorectal cancers. Ann Thorac Surg. 1998; 66:214–218. PMID: 9692467.
Article4. Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994; 343:1405–1410. PMID: 7515134.
Article5. Kumar R, Price TJ, Beeke C, Jain K, Patel G, Padbury R, et al. Colorectal cancer survival: an analysis of patients with metastatic disease synchronous and metachronous with the primary tumor. Clin Colorectal Cancer. 2014; 13:87–93. PMID: 24373733.
Article6. Konopke R, Kersting S, Makowiec F, Gassmann P, Kuhlisch E, Senninger N, et al. Resection of colorectal liver metastases: is a resection margin of 3 mm enough?: a multicenter analysis of the GAST Study Group. World J Surg. 2008; 32:2047–2056. PMID: 18521661.7. Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008; 143:384–393. PMID: 18291260.
Article8. Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013; 11:141–152. PMID: 23411381.9. Brouquet A, Vauthey JN, Contreras CM, Walsh GL, Vaporciyan AA, Swisher SG, et al. Improved survival after resection of liver and lung colorectal metastases compared with liver-only metastases: a study of 112 patients with limited lung metastatic disease. J Am Coll Surg. 2011; 213:62–69. PMID: 21700179.
Article10. Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006; 202:468–475. PMID: 16500252.
Article11. Arnaud JP, Dumont P, Adloff M, Leguillou A, Py JM. Natural history of colorectal carcinoma with untreated liver metastases. Surg Gastroenterol. 1984; 3:37–42. PMID: 6522907.12. D'Angelica MI, Correa-Gallego C, Paty PB, Cercek A, Gewirtz AN, Chou JF, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015; 261:353–360. PMID: 24646562.13. Helling TS, Martin M. Cause of death from liver metastases in colorectal cancer. Ann Surg Oncol. 2014; 21:501–506. PMID: 24081807.
Article14. Sourrouille I, Mordant P, Maggiori L, Dokmak S, Lesèche G, Panis Y, et al. Long-term survival after hepatic and pulmonary resection of colorectal cancer metastases. J Surg Oncol. 2013; 108:220–224. PMID: 23893480.
Article15. Ahmed S, Johnson K, Ahmed O, Iqbal N. Advances in the management of colorectal cancer: from biology to treatment. Int J Colorectal Dis. 2014; 29:1031–1042. PMID: 24953060.
Article16. Headrick JR, Miller DL, Nagorney DM, Allen MS, Deschamps C, Trastek VF, et al. Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg. 2001; 71:975–979. PMID: 11269484.
Article17. Miller G, Biernacki P, Kemeny NE, Gonen M, Downey R, Jarnagin WR, et al. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg. 2007; 205:231–238. PMID: 17660069.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simultaneous Laparoscopy-Assisted Resection for Colorectal Cancer and Metastases
- Risk Stratification of T1 Colorectal Cancer Metastasis to Lymph Nodes: Current Status and Perspective
- Surgical Resection for Lung Metastases from Colorectal Cancer
- Surgical resection of synchronous and metachronous lung and liver metastases of colorectal cancers
- Oncologic outcomes following metastasectomy in colorectal cancer patients developing distant metastases after initial treatment