Stromal Expression of MicroRNA-21 in Advanced Colorectal Cancer Patients with Distant Metastases
- Affiliations
-
- 1Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea. hye2@snu.ac.kr
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2345549
- DOI: http://doi.org/10.4132/jptm.2016.03.19
Abstract
- BACKGROUND
The aim of this study was to determine the regional heterogeneity and clinicopathological significance of microRNA-21 (miR-21) in advanced colorectal cancer (CRC) patients with distant metastasis.
METHODS
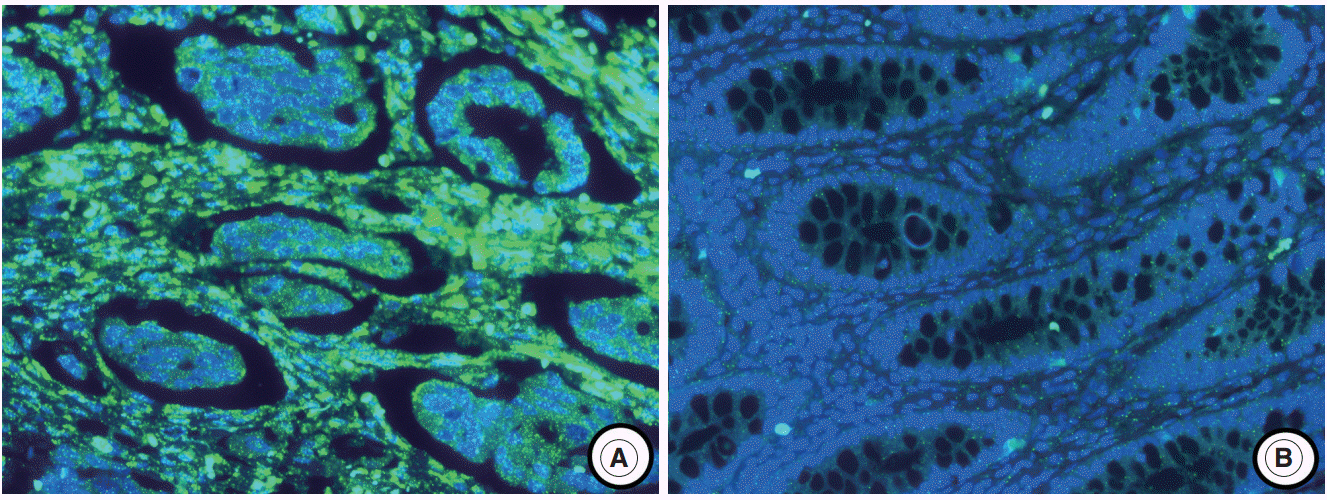
miR-21 expression was investigated by using locked nucleic acid- fluorescence in situ hybridization in the center and periphery of the primary cancer and in distant metastasis from 170 patients with advanced CRC. In addition, α-smooth muscle actin and desmin were evaluated to identify cancer-associated fibroblasts (CAFs) by using immunohistochemistry.
RESULTS
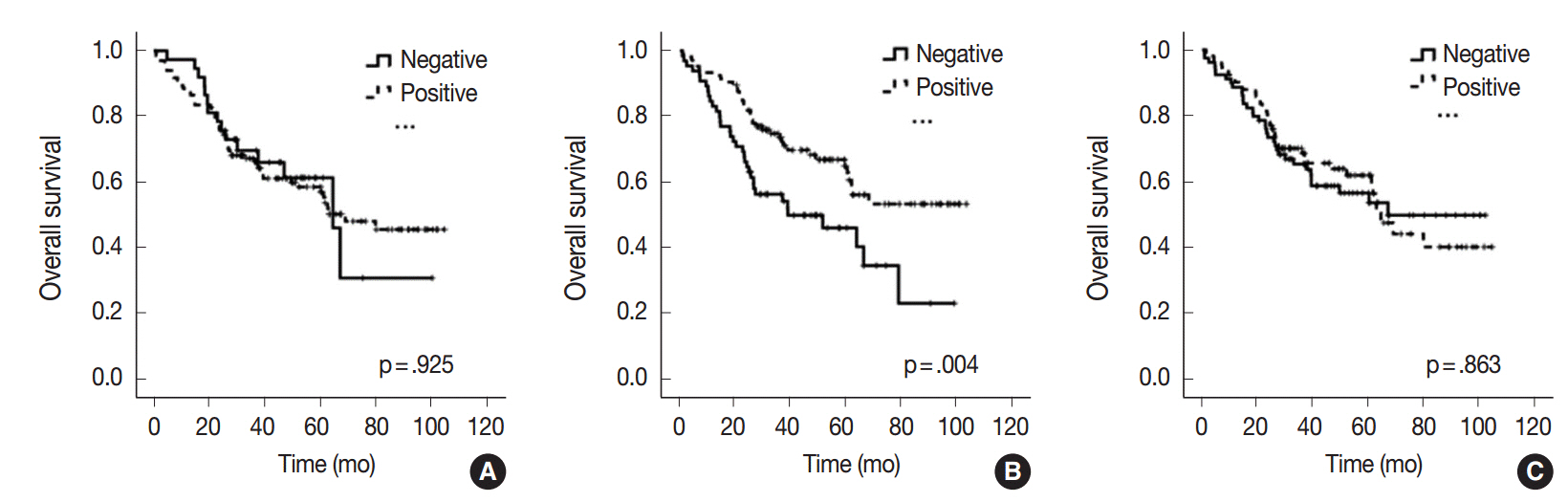
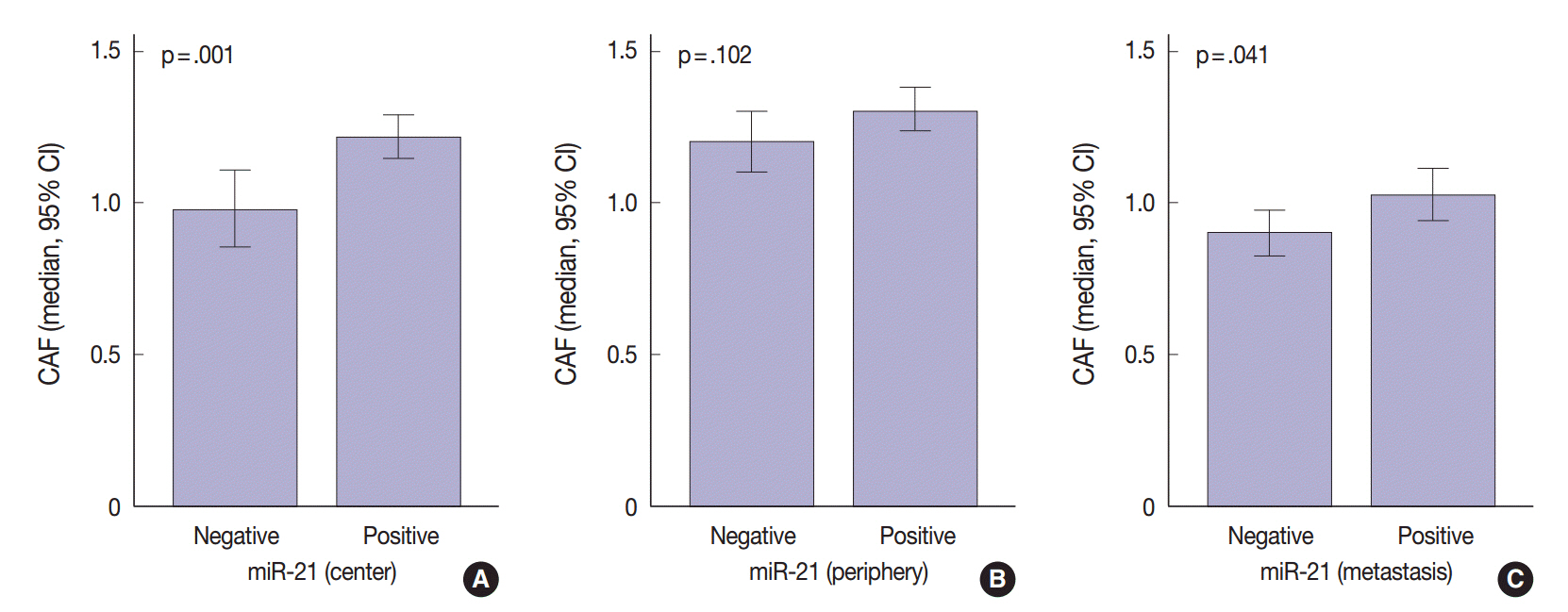
The miR-21 signal was observed in the cancer stroma. The expression of miR-21 (a score of 1-4) in the center and periphery of the primary cancer and in distant metastasis was observed in specimens from 133 (78.2%), 105 (61.8%), and 91 (53.5%) patients, respectively. miR-21 expression was heterogeneous in advanced CRC. Discordance between miR-21 expression in the center of the primary cancer and either the periphery of the primary cancer or distant metastasis was 31.7% or 44.7%, respectively. miR-21 stromal expression in the periphery of the primary cancer was significantly associated with a better prognosis (p=.004). miR-21 expression was significantly associated with CAFs in the center of the primary cancer (p=.001) and distant metastases (p=.041).
CONCLUSIONS
miR-21 expression is observed in cancer stroma related to the CAF quantity and frequently presents regional heterogeneity in CRC. Our findings indicate that the role of miR-21 in predicting prognosis may be controversial but provide a new perspective of miR-21 level measurement in cancer specimens.
MeSH Terms
Figure
Cited by 4 articles
-
Prognostic Utility of Histological Growth Patterns of Colorectal Lung Oligometastasis
Son Jae Yeong, Min Gyoung Pak, Hyoun Wook Lee, Seung Yeon Ha, Mee Sook Roh
J Pathol Transl Med. 2018;52(2):98-104. doi: 10.4132/jptm.2017.12.27.MicroRNA-552 expression in colorectal cancer and its clinicopathological significance
Joon Im, Soo Kyung Nam, Hye Seung Lee
J Pathol Transl Med. 2021;55(2):125-131. doi: 10.4132/jptm.2021.01.17.Ligand-Independent Epidermal Growth Factor Receptor Overexpression Correlates with Poor Prognosis in Colorectal Cancer
Sumi Yun, Yoonjin Kwak, Soo Kyung Nam, An Na Seo, Heung-Kwon Oh, Duck-Woo Kim, Sung-Bum Kang, Hye Seung Lee
Cancer Res Treat. 2018;50(4):1351-1361. doi: 10.4143/crt.2017.487.High-Throughput Multiplex Immunohistochemical Imaging of the Tumor and Its Microenvironment
Jiwon Koh, Yoonjin Kwak, Jin Kim, Woo Ho Kim
Cancer Res Treat. 2020;52(1):98-108. doi: 10.4143/crt.2019.195.
Reference
-
1. Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003; 301:336–8.
Article2. Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007; 302:1–12.
Article3. Kwak PB, Iwasaki S, Tomari Y. The microRNA pathway and cancer. Cancer Sci. 2010; 101:2309–15.
Article4. Chan SH, Wu CW, Li AF, Chi CW, Lin WC. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res. 2008; 28:907–11.5. Gabriely G, Wurdinger T, Kesari S, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008; 28:5369–80.
Article6. Qian B, Katsaros D, Lu L, et al. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-beta1. Breast Cancer Res Treat. 2009; 117:131–40.7. Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Georgoulias V, Lianidou ES. Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem. 2008; 54:1696–704.
Article8. Zhang A, Liu Y, Shen Y, Xu Y, Li X. miR-21 modulates cell apoptosis by targeting multiple genes in renal cell carcinoma. Urology. 2011; 78:474.
Article9. Hiyoshi Y, Kamohara H, Karashima R, et al. MicroRNA-21 regulates the proliferation and invasion in esophageal squamous cell carcinoma. Clin Cancer Res. 2009; 15:1915–22.
Article10. Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008; 27:2128–36.
Article11. Lu Z, Liu M, Stribinskis V, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008; 27:4373–9.
Article12. Mathé EA, Nguyen GH, Bowman ED, et al. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res. 2009; 15:6192–200.
Article13. Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008; 14:2348–60.
Article14. Yu Y, Nangia-Makker P, Farhana L, Rajendra SG, Levi E, Majumdar AP. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol Cancer. 2015; 14:98.
Article15. Kanaan Z, Rai SN, Eichenberger MR, et al. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012; 256:544–51.16. Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009; 58:1375–81.
Article17. Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010; 127:118–26.
Article18. Yu Y, Sarkar FH, Majumdar AP. Down-regulation of miR-21 induces differentiation of chemoresistant colon cancer cells and enhances susceptibility to therapeutic regimens. Transl Oncol. 2013; 6:180–6.
Article19. Valeri N, Gasparini P, Braconi C, et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2). Proc Natl Acad Sci U S A. 2010; 107:21098–103.
Article20. Xia X, Yang B, Zhai X, et al. Prognostic role of microRNA-21 in colorectal cancer: a meta-analysis. PLoS One. 2013; 8:e80426.
Article21. Kjaer-Frifeldt S, Hansen TF, Nielsen BS, et al. The prognostic importance of miR-21 in stage II colon cancer: a population-based study. Br J Cancer. 2012; 107:1169–74.
Article22. Kang WK, Lee JK, Oh ST, Lee SH, Jung CK. Stromal expression of miR-21 in T3-4a colorectal cancer is an independent predictor of early tumor relapse. BMC Gastroenterol. 2015; 15:2.
Article23. Zhang JX, Song W, Chen ZH, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013; 14:1295–306.
Article24. Nielsen BS, Jørgensen S, Fog JU, et al. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis. 2011; 28:27–38.
Article25. Kwak Y, Lee HE, Kim WH, Kim DW, Kang SB, Lee HS. The clinical implication of cancer-associated microvasculature and fibroblast in advanced colorectal cancer patients with synchronous or metachronous metastases. PLoS One. 2014; 9:e91811.
Article26. Tsujino T, Seshimo I, Yamamoto H, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007; 13:2082–90.
Article27. Trimboli AJ, Cantemir-Stone CZ, Li F, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009; 461:1084–91.
Article28. Knijn N, Tol J, Punt CJ. Current issues in the targeted therapy of advanced colorectal cancer. Discov Med. 2010; 9:328–36.29. O’Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008; 26:2336–41.30. Albanese I, Scibetta AG, Migliavacca M, et al. Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem Biophys Res Commun. 2004; 325:784–91.
Article31. Park JH, Han SW, Oh DY, et al. Analysis of KRAS, BRAF, PTEN, IGF1R, EGFR intron 1 CA status in both primary tumors and paired metastases in determining benefit from cetuximab therapy in colon cancer. Cancer Chemother Pharmacol. 2011; 68:1045–55.
Article32. Baldus SE, Schaefer KL, Engers R, Hartleb D, Stoecklein NH, Gabbert HE. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res. 2010; 16:790–9.
Article33. Villegas-Ruiz V, Juárez-Méndez S, Pérez-González OA, et al. Heterogeneity of microRNAs expression in cervical cancer cells: over-expression of miR-196a. Int J Clin Exp Pathol. 2014; 7:1389–401.34. Jepsen RK, Novotny GW, Klarskov LL, Christensen IJ, Riis LB, Høgdall E. Intra-tumor heterogeneity of microRNA-92a, microRNA-375 and microRNA-424 in colorectal cancer. Exp Mol Pathol. 2016; 100:125–31.
Article35. Raychaudhuri M, Schuster T, Buchner T, et al. Intratumoral heterogeneity of microRNA expression in breast cancer. J Mol Diagn. 2012; 14:376–84.
Article36. Bullock MD, Pickard KM, Nielsen BS, et al. Pleiotropic actions of miR-21 highlight the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis. 2013; 4:e684.
Article37. Yamamichi N, Shimomura R, Inada K, et al. Locked nucleic acid in situ hybridization analysis of miR-21 expression during colorectal cancer development. Clin Cancer Res. 2009; 15:4009–16.
Article38. Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009; 28:369–78.
Article39. Fassan M, Pizzi M, Giacomelli L, et al. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Arch. 2011; 458:413–9.
Article40. Aprelikova O, Green JE. MicroRNA regulation in cancer-associated fibroblasts. Cancer Immunol Immunother. 2012; 61:231–7.
Article41. Lou E, Subramanian S, Steer CJ. Pancreatic cancer: modulation of KRAS, microRNAs, and intercellular communication in the setting of tumor heterogeneity. Pancreas. 2013; 42:1218–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Expression of DCC Protein in Colorectal Carcinoma
- Distant Metastasis Identified Immediately after Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer
- Expression of E-cadherin and beta-catenin in Stage II Colorectal Cancer Patients with Metachronous Distant Metastasis
- Prognostic Value of HSP 70 in Colorectal Cancer
- Management of Colorectal Cancer Liver Metastasis