J Korean Med Sci.
2015 Sep;30(9):1246-1252. 10.3346/jkms.2015.30.9.1246.
Age-related NADPH Oxidase (arNOX) Activity Correlated with Cartilage Degradation and Bony Changes in Age-related Osteoarthritis
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Yeungnam University, Daegu, Korea. yhhong@med.yu.ac.kr
- 2Department of Physiology, College of Medicine, Yeungnam University, Daegu, Korea.
- 3Department of Orthopedic Surgery, College of Medicine, Yeungnam University, Daegu, Korea.
- 4Department of Anatomy, College of Medicine, Yeungnam University, Daegu, Korea.
- KMID: 2344148
- DOI: http://doi.org/10.3346/jkms.2015.30.9.1246
Abstract
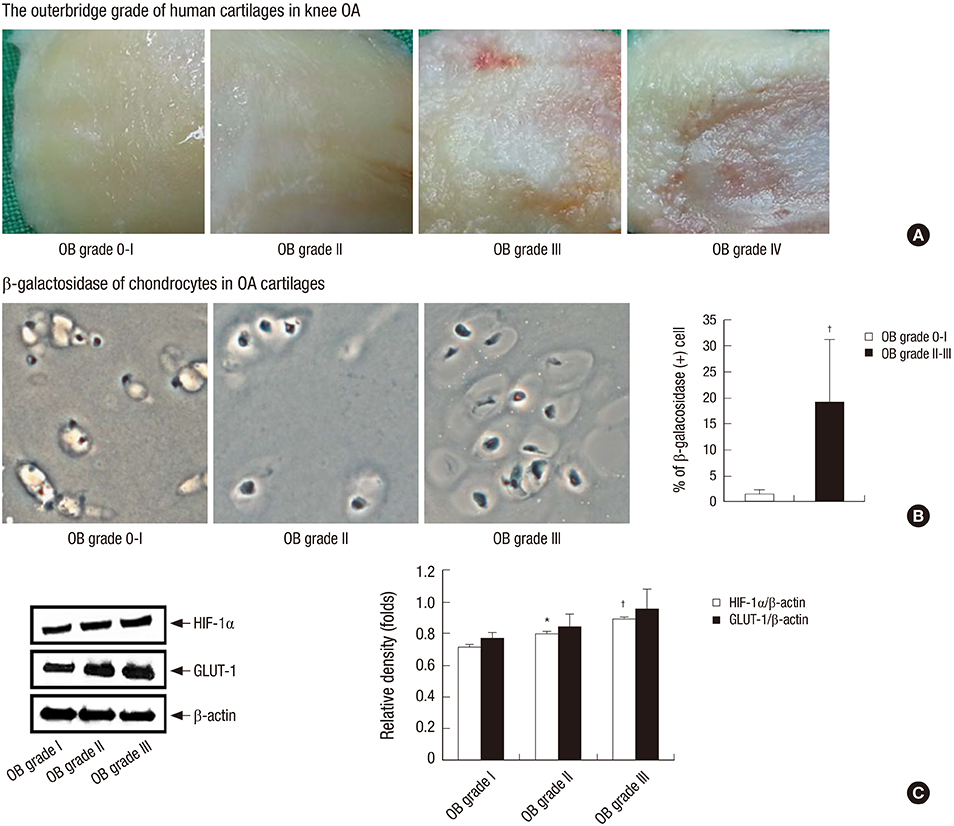
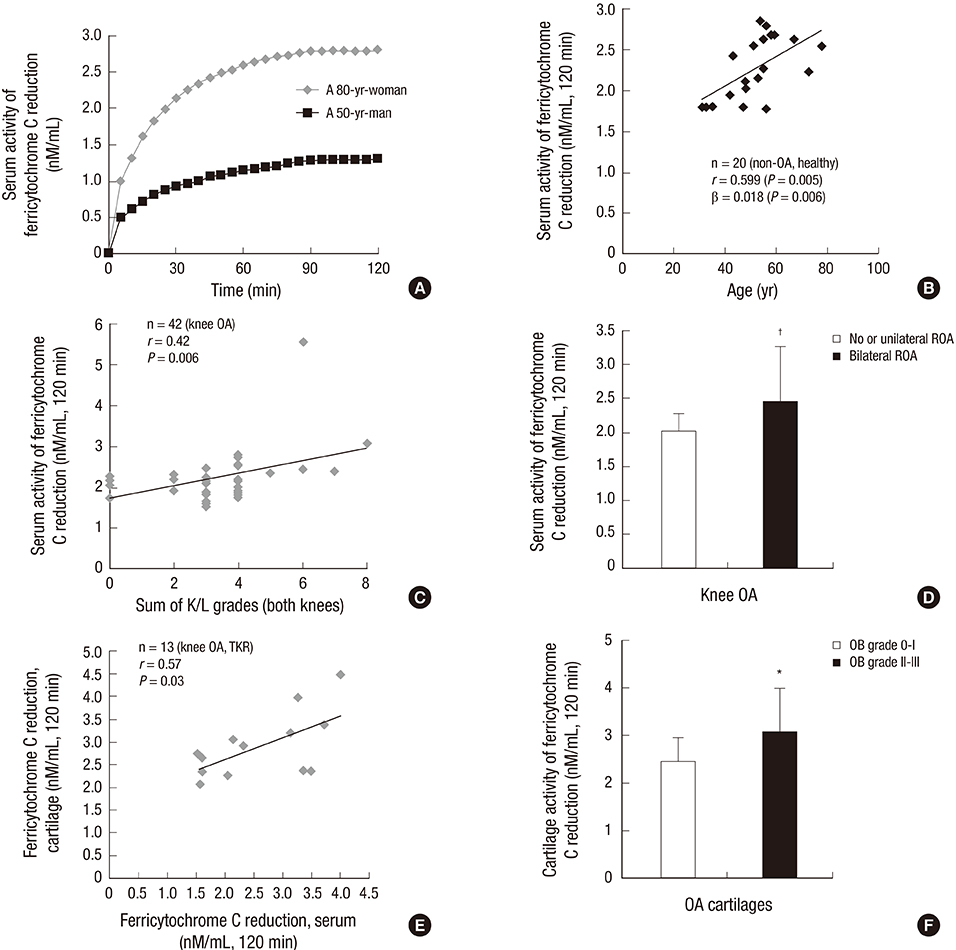
- The purpose of this study was to investigate the age-related NADPH oxidase (arNOX) activity in patients with age-related knee osteoarthritis (OA). Serum and cartilage arNOX activities were determined using an oxidized ferricytochrome C reduction assay. Full-thickness knee joint cartilages obtained through total knee replacement surgery were graded according to the Outerbridge (OB) classification. Radiographic severity of OA was determined on Knee X-rays according to the Kellgren-Lawrence (K/L) grading system. Cartilage beta-galactosidase, HIF-1alpha, and GLUT-1 expression levels were evaluated as markers for tissue senescence, hypoxia, and glycolysis. Higher arNOX activities occurred with higher levels of cartilage beta-galactosidase, HIF-1alpha, and GLUT-1 (P = 0.002). arNOX activity in cartilages with surface defects (OB grade II, III) was higher than in those without the defects (OB grade 0, I) (P = 0.012). Cartilage arNOX activity showed a positive correlation with serum arNOX activity (r = -0.577, P = 0.023). Serum arNOX activity was significantly higher in the OA subgroup with bilateral ROA than in the OA with no or unilateral ROA (2.449 +/- 0.81, 2.022 +/- 0.251 nM/mL, respectively, P = 0.019). The results of this study demonstrate that OA itself is not a cause to increase arNOX activities, however, arNOX hyperactivity is related to a high degree of cartilage degradation, and a high grade and extent of ROA in age-related OA.
Keyword
MeSH Terms
-
Biomarkers/metabolism
Cartilage Diseases/*enzymology
Cartilage, Articular/*enzymology
Enzyme Activation
Female
Humans
Male
Middle Aged
NADH, NADPH Oxidoreductases
Osteoarthritis, Knee/*diagnosis/*enzymology
Osteoporosis/*diagnosis/*enzymology
Reproducibility of Results
Sensitivity and Specificity
Statistics as Topic
Biomarkers
NADH, NADPH Oxidoreductases
Figure
Reference
-
1. Di Cesare PE, Haudenschild DR, Samuels J, Abramson SB, Firestein GS. Pathogenesis of osteoarthritis. In : Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, Kelley WN, editors. Kelley's textbook of rheumatology. 9th ed. Philadelphia, PA: Elsevier/Saunders;2013. p. 1617–1633.2. van der Kraan PM. Osteoarthritis year 2012 in review: biology. Osteoarthritis Cartilage. 2012; 20:1447–1450.3. Peansukmanee S, Vaughan-Thomas A, Carter SD, Clegg PD, Taylor S, Redmond C, Mobasheri A. Effects of hypoxia on glucose transport in primary equine chondrocytes in vitro and evidence of reduced GLUT1 gene expression in pathologic cartilage in vivo. J Orthop Res. 2009; 27:529–535.4. Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. 2011; 23:492–496.5. Morré DJ, Chueh PJ, Lawler J, Morré DM. The sulfonylurea-inhibited NADH oxidase activity of HeLa cell plasma membranes has properties of a protein disulfide-thiol oxidoreductase with protein disulfide-thiol interchange activity. J Bioenerg Biomembr. 1998; 30:477–487.6. Morré DM, Lenaz G, Morré DJ. Surface oxidase and oxidative stress propagation in aging. J Exp Biol. 2000; 203:1513–1521.7. Morré DJ, Morré DM. Cell surface NADH oxidases (ECTO-NOX proteins) with roles in cancer, cellular time-keeping, growth, aging and neurodegenerative diseases. Free Radic Res. 2003; 37:795–808.8. Morré DJ, Morré DM. ECTO-NOX proteins growth, cancer, and aging. New York, NY: Springer;2013.9. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961; 43-B:752–757.10. Butler J, Koppenol WH, Margoliash E. Kinetics and mechanism of the reduction of ferricytochrome c by the superoxide anion. J Biol Chem. 1982; 257:10747–10750.11. Morré DM, Morré DJ. Specificity of coenzyme Q inhibition of an aging-related cell surface NADH oxidase (ECTO-NOX) that generates superoxide. Biofactors. 2003; 18:33–43.12. Morré DM, Meadows C, Hostetler B, Weston N, Kern D, Draelos Z, Morré DJ. Age-related ENOX protein (arNOX) activity correlated with oxidative skin damage in the elderly. Biofactors. 2008; 34:237–244.13. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957; 16:494–502.14. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995; 92:9363–9367.15. Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002; 1:57–65.16. Pfander D, Cramer T, Swoboda B. Hypoxia and HIF-1alpha in osteoarthritis. Int Orthop. 2005; 29:6–9.17. Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001; 15:2865–2876.18. Hong YH, Park CW, Kim HS, Won KC, Kim YW, Lee CK. Effects of hypoxia/ischemia on catabolic mediators of cartilage in a human chondrocyte, SW1353. Biochem Biophys Res Commun. 2013; 431:478–483.19. Ren BF, Deng LF, Wang J, Zhu YP, Wei L, Zhou Q. Hypoxia regulation of facilitated glucose transporter-1 and glucose transporter-3 in mouse chondrocytes mediated by HIF-1alpha. Joint Bone Spine. 2008; 75:176–181.20. Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 1997; 77:425–464.21. Morré DM, Meadows C, Morré DJ. arNOX: generator of reactive oxygen species in the skin and sera of aging individuals subject to external modulation. Rejuvenation Res. 2010; 13:162–164.22. Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008; 10:179–206.23. Morré DJ, Morré DM. Aging-related cell surface ECTO-NOX protein, arNOX, a preventive target to reduce atherogenic risk in the elderly. Rejuvenation Res. 2006; 9:231–236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NADPH oxidase mediated oxidative stress in hepatic fibrogenesis
- Clinical Usefulness of Uric Acid as a Biomarker for Knee Osteoarthritis: A Comparative Analysis With Plain Radiography and Musculoskeletal Ultrasound
- Ultrasonography in Osteoarthritis
- Cilostazol Attenuates 4-hydroxynonenal-enhanced CD36 Expression on Murine Macrophages via Inhibition of NADPH Oxidase-derived Reactive Oxygen Species Production
- Lignans with NADPH Oxidase 2 (NOX2)-inhibitory Activity from the Fruits of Schisandra chinensis