J Periodontal Implant Sci.
2015 Apr;45(2):38-45. 10.5051/jpis.2015.45.2.38.
The effect of photodynamic therapy on Aggregatibacter actinomycetemcomitans attached to surface-modified titanium
- Affiliations
-
- 1Department of Periodontology, Research Institute for Oral Sciences, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea. periojk@gwnu.ac.kr
- 2Department of Oral Microbiology, Research Institute for Oral Sciences, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea.
- KMID: 2329654
- DOI: http://doi.org/10.5051/jpis.2015.45.2.38
Abstract
- PURPOSE
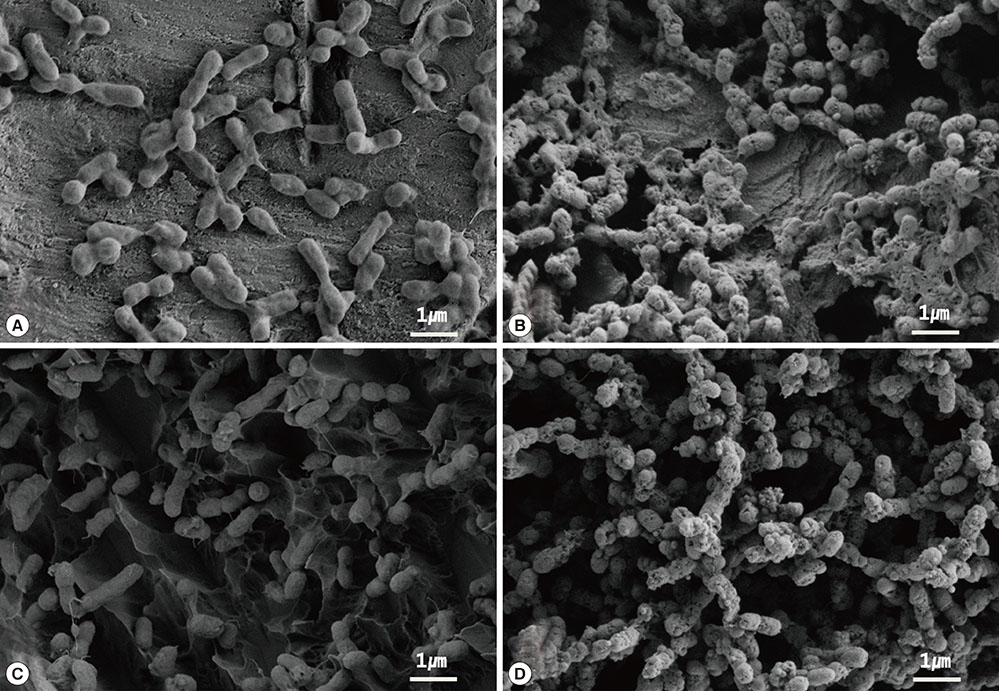
The purpose of this study was to evaluate the effect of photodynamic therapy (PDT) using erythrosine and a green light emitting diode (LED) light source on biofilms of Aggregatibacter actinomycetemcomitans attached to resorbable blasted media (RBM) and sandblasted, large-grit, acid-etched (SLA) titanium surfaces in vitro.
METHODS
RBM and SLA disks were subdivided into four groups, including one control group and three test groups (referred to as E0, E30, E60), in order to evaluate the effect of PDT on each surface. The E0 group was put into 500 microL of 20 microM erythrosine for 60 seconds without irradiation, the E30 group was put into erythrosine for 60 seconds and was then irradiated with a LED for 30 seconds, and the E60 group was put into erythrosine for 60 seconds and then irradiated with a LED for 60 seconds. After PDT, sonication was performed in order to detach the bacteria, the plates were incubated under anaerobic conditions on brucella blood agar plates for 72 hours at 37degrees C, and the number of colony-forming units (CFUs) was determined.
RESULTS
Significant differences were found between the control group and the E30 and E60 groups (P<0.05). A significantly lower quantity of CFU/mL was found in the E30 and E60 groups on both titanium disk surfaces. In confocal scanning laser microscopy images, increased bacterial death was observed when disks were irradiated for a longer period of time.
CONCLUSIONS
These findings suggest that PDT using erythrosine and a green LED is effective in reducing the viability of A. actinomycetemcomitans attached to surface-modified titanium in vitro.
MeSH Terms
Figure
Cited by 1 articles
-
티타늄 표면에 형성한
Staphylococcus aureus 바이오필름에 대한 인도시아닌 그린을 활용한 광역학치료의 항미생물 효과
Seung Gi Kim, Si-Young Lee, Jong-Bin Lee, Heung-Sik Um, Jae-Kwan Lee
J Dent Rehabil Appl Sci. 2024;40(2):55-63. doi: 10.14368/jdras.2024.40.2.55.
Reference
-
1. Takasaki AA, Aoki A, Mizutani K, Schwarz F, Sculean A, Wang CY, et al. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000. 2009; 51:109–140.
Article2. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998; 17:63–76.
Article3. Rams TE, Link CC Jr. Microbiology of failing dental implants in humans: electron microscopic observations. J Oral Implantol. 1983; 11:93–100.4. Mombelli A, van Oosten MA, Schürch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987; 2:145–151.
Article5. Becker W, Becker BE, Newman MG, Nyman S. Clinical and microbiologic findings that may contribute to dental implant failure. Int J Oral Maxillofac Implants. 1990; 5:31–38.6. Sanz M, Newman MG, Nachnani S, Holt R, Stewart R, Flemmig T. Characterization of the subgingival microbial flora around endosteal sapphire dental implants in partially edentulous patients. Int J Oral Maxillofac Implants. 1990; 5:247–253.7. Alcoforado GA, Rams TE, Feik D, Slots J. Microbial aspects of failing osseointegrated dental implants in humans. J Parodontol. 1991; 10:11–18.8. Sbordone L, Barone A, Ramaglia L, Ciaglia RN, Iacono VJ. Antimicrobial susceptibility of periodontopathic bacteria associated with failing implants. J Periodontol. 1995; 66:69–74.
Article9. Mombelli A, Marxer M, Gaberthüel T, Grunder U, Lang NP. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 1995; 22:124–130.
Article10. Persson LG, Berglundh T, Lindhe J, Sennerby L. Re-osseointegration after treatment of peri-implantitis at different implant surfaces. An experimental study in the dog. Clin Oral Implants Res. 2001; 12:595–603.
Article11. Lang NP, Wilson TG, Corbet EF. Biological complications with dental implants: their prevention, diagnosis and treatment. Clin Oral Implants Res. 2000; 11:Suppl 1. 146–155.
Article12. Matarasso S, Quaremba G, Coraggio F, Vaia E, Cafiero C, Lang NP. Maintenance of implants: an in vitro study of titanium implant surface modifications subsequent to the application of different prophylaxis procedures. Clin Oral Implants Res. 1996; 7:64–72.
Article13. Kreisler M, Kohnen W, Marinello C, Götz H, Duschner H, Jansen B, et al. Bactericidal effect of the Er:YAG laser on dental implant surfaces: an in vitro study. J Periodontol. 2002; 73:1292–1298.
Article14. Takasaki AA, Aoki A, Mizutani K, Kikuchi S, Oda S, Ishikawa I. Er:YAG laser therapy for peri-implant infection: a histological study. Lasers Med Sci. 2007; 22:143–157.
Article15. Kreisler M, Götz H, Duschner H. Effect of Nd:YAG, Ho:YAG, Er:YAG, CO2, and GaAIAs laser irradiation on surface properties of endosseous dental implants. Int J Oral Maxillofac Implants. 2002; 17:202–211.16. Mouhyi J, Sennerby L, Nammour S, Guillaume P, Van Reck J. Temperature increases during surface decontamination of titanium implants using CO2 laser. Clin Oral Implants Res. 1999; 10:54–61.
Article17. Oyster DK, Parker WB, Gher ME. CO2 lasers and temperature changes of titanium implants. J Periodontol. 1995; 66:1017–1024.18. Romanos GE, Everts H, Nentwig GH. Effects of diode and Nd:YAG laser irradiation on titanium discs: a scanning electron microscope examination. J Periodontol. 2000; 71:810–815.
Article19. Haas R, Dörtbudak O, Mensdorff-Pouilly N, Mailath G. Elimination of bacteria on different implant surfaces through photosensitization and soft laser. An in vitro study. Clin Oral Implants Res. 1997; 8:249–254.
Article20. Tawakoli PN, Al-Ahmad A, Hoth-Hannig W, Hannig M, Hannig C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin Oral Investig. 2013; 17:841–850.
Article21. Mombelli A. Etiology, diagnosis, and treatment considerations in peri-implantitis. Curr Opin Periodontol. 1997; 4:127–136.22. Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res. 2001; 12:104–108.
Article23. Bürgers R, Gerlach T, Hahnel S, Schwarz F, Handel G, Gosau M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res. 2010; 21:156–164.24. Song KH, Kim IK, Jang KS, Kim KN, Choi JU. Histomorphometric study of dental implants with RBM and SLA surface in the rabbit tibia. J Korean Assoc Oral Maxillofac Surg. 2006; 32:514–523.25. Marotti J, Tortamano P, Cai S, Ribeiro MS, Franco JE, de Campos TT. Decontamination of dental implant surfaces by means of photodynamic therapy. Lasers Med Sci. 2013; 28:303–309.
Article26. Shibli JA, Martins MC, Nociti FH Jr, Garcia VG, Marcantonio E Jr. Treatment of ligature-induced peri-implantitis by lethal photosensitization and guided bone regeneration: a preliminary histologic study in dogs. J Periodontol. 2003; 74:338–345.
Article27. Shibli JA, Martins MC, Theodoro LH, Lotufo RF, Garcia VG, Marcantonio EJ. Lethal photosensitization in microbiological treatment of ligature-induced peri-implantitis: a preliminary study in dogs. J Oral Sci. 2003; 45:17–23.
Article28. Shibli JA, Martins MC, Ribeiro FS, Garcia VG, Nociti FH Jr, Marcantonio E Jr. Lethal photosensitization and guided bone regeneration in treatment of peri-implantitis: an experimental study in dogs. Clin Oral Implants Res. 2006; 17:273–281.
Article29. Haas R, Baron M, Dörtbudak O, Watzek G. Lethal photosensitization, autogenous bone, and e-PTFE membrane for the treatment of peri-implantitis: preliminary results. Int J Oral Maxillofac Implants. 2000; 15:374–382.30. Begue WJ, Bard RC, Koehne GW. Microbial inhibition by erythrosin. J Dent Res. 1966; 45:1464–1467.
Article31. Baab DA, Broadwell AH, Williams BL. A comparison of antimicrobial activity of four disclosant dyes. J Dent Res. 1983; 62:837–841.
Article32. Marsh PD, Bevis RA, Newman HN, Hallsworth AS, Robinson C, Weatherell JA, et al. Antibacterial activity of some plaque-disclosing agents and dyes. Caries Res. 1989; 23:348–350.
Article33. Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006; 57:680–684.
Article34. Metcalf D, Robinson C, Devine D, Wood S. Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J Antimicrob Chemother. 2006; 58:190–192.
Article35. Lee SY, Chang BS, Um HS, Ma DS. Comparison of photodynamic bactericidal effects of erythrosine against Streptococcus mutans and Streptococcus sobrinus by different wavelength of LED lights. J Korean Acad Oral Health. 2012; 36:20–25.36. Qin Y, Luan X, Bi L, He G, Bai X, Zhou C, et al. Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques. Lasers Med Sci. 2008; 23:49–54.
Article37. Geminiani A, Caton JG, Romanos GE. Temperature change during non-contact diode laser irradiation of implant surfaces. Lasers Med Sci. 2012; 27:339–342.
Article38. Eriksson RA, Albrektsson T. The effect of heat on bone regeneration: an experimental study in the rabbit using the bone growth chamber. J Oral Maxillofac Surg. 1984; 42:705–711.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of osteoclastogenesis-inducing cytokines and invasion by alive Aggregatibacter actinomycetemcomitans in osteoblasts
- Effects of Sub Minimal Inhibitory Concentration of Metronidazole and Penicillin on Morphology of Aggregatibacter actinomycetemcomitans: Scanning Electron Microscopy Observation
- Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties
- The effect of Actinobacillus actinomycetemcomitans lipopolysaccharide on rat periodontal tissues
- Induction of IL-8 and reactive oxygen species in periodontal ligament cells by Aggregatibacter actinomycetemcomitans