Korean J Orthod.
2017 Jan;47(1):3-10. 10.4041/kjod.2017.47.1.3.
Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties
- Affiliations
-
- 1Department of Orthodontics, School of Dentistry, Kyungpook National University, Daegu, Korea. hmkyung@knu.ac.kr
- 2Department of Chemistry Education, Kyungpook National University, Daegu, Korea.
- 3Research Institute of Advanced Energy Technology, Kyungpook National University, Daegu, Korea.
- 4Department of Nanoscience and Nanotechnology, Kyungpook National University, Daegu, Korea.
- 5Department of Oral Microbiology and Immunology, Kyungpook National University, Daegu, Korea.
- KMID: 2392191
- DOI: http://doi.org/10.4041/kjod.2017.47.1.3
Abstract
OBJECTIVE
Microbial aggregation around dental implants can lead to loss/loosening of the implants. This study was aimed at surface treating titanium microimplants with silver nanoparticles (AgNPs) to achieve antibacterial properties.
METHODS
AgNP-modified titanium microimplants (Ti-nAg) were prepared using two methods. The first method involved coating the microimplants with regular AgNPs (Ti-AgNP) and the second involved coating them with a AgNP-coated biopolymer (Ti-BP-AgNP). The topologies, microstructures, and chemical compositions of the surfaces of the Ti-nAg were characterized by scanning electron microscopy (SEM) equipped with energy-dispersive spectrometer (EDS) and X-ray photoelectron spectroscopy (XPS). Disk diffusion tests using Streptococcus mutans, Streptococcus sanguinis, and Aggregatibacter actinomycetemcomitans were performed to test the antibacterial activity of the Ti-nAg microimplants.
RESULTS
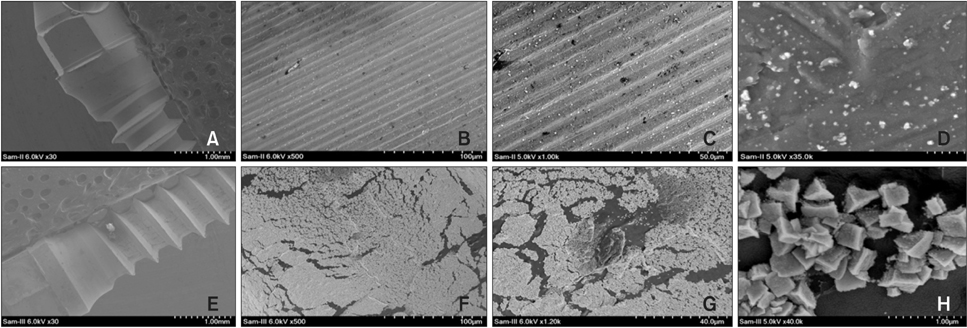
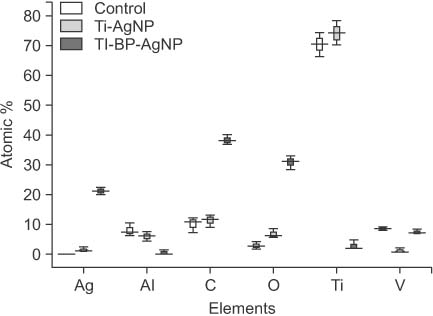
SEM revealed that only a meager amount of AgNPs was sparsely deposited on the Ti-AgNP surface with the first method, while a layer of AgNP-coated biopolymer extended along the Ti-BP-AgNP surface in the second method. The diameters of the coated nanoparticles were in the range of 10 to 30 nm. EDS revealed 1.05 atomic % of Ag on the surface of the Ti-AgNP and an astounding 21.2 atomic % on the surface of the Ti-BP-AgNP. XPS confirmed the metallic state of silver on the Ti-BP-AgNP surface. After 24 hours of incubation, clear zones of inhibition were seen around the Ti-BP-AgNP microimplants in all three test bacterial culture plates, whereas no antibacterial effect was observed with the Ti-AgNP microimplants.
CONCLUSIONS
Titanium microimplants modified with Ti-BP-AgNP exhibit excellent antibacterial properties, making them a promising implantable biomaterial.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Antibacterial and remineralization effects of orthodontic bonding agents containing bioactive glass
You-Min Kim, Dong-Hyun Kim, Chang Weon Song, Seog-Young Yoon, Se-Yeon Kim, Hee Sam Na, Jin Chung, Yong-Il Kim, Yong Hoon Kwon
Korean J Orthod. 2018;48(3):163-171. doi: 10.4041/kjod.2018.48.3.163.The effect of fluoride-containing oral rinses on the corrosion resistance of titanium alloy (Ti-6Al-4V)
Gui-Yue Huang, Heng Bo Jiang, Jung-Yul Cha, Kwang-Mahn Kim, Chung-Ju Hwang
Korean J Orthod. 2017;47(5):306-312. doi: 10.4041/kjod.2017.47.5.306.
Reference
-
1. Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007; 23:844–854.
Article2. Schierholz JM, Beuth J. Implant infections: a haven for opportunistic bacteria. J Hosp Infect. 2001; 49:87–93.
Article3. Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res. 2002; 13:1–19.
Article4. Zitzmann NU, Berglundh T, Ericsson I, Lindhe J. Spontaneous progression of experimentally induced periimplantitis. J Clin Periodontol. 2004; 31:845–849.
Article5. Ericsson I, Berglundh T, Marinello C, Liljenberg B, Lindhe J. Long-standing plaque and gingivitis at implants and teeth in the dog. Clin Oral Implants Res. 1992; 3:99–103.
Article6. Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008; 35:8 Suppl. 282–285.
Article7. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008; 35:8 Suppl. 286–291.
Article8. Wadström T. Molecular aspects of bacterial adhesion, colonization, and development of infections associated with biomaterials. J Invest Surg. 1989; 2:353–360.
Article9. Liao J, Anchun M, Zhu Z, Quan Y. Antibacterial titanium plate deposited by silver nanoparticles exhibits cell compatibility. Int J Nanomedicine. 2010; 5:337–342.10. Meredith DO, Eschbach L, Riehle MO, Curtis AS, Richards RG. Microtopography of metal surfaces influence fibroblast growth by modifying cell shape, cytoskeleton, and adhesion. J Orthop Res. 2007; 25:1523–1533.
Article11. Chang YY, Huang HL, Lai CH, Hsu JT, Shieh TM, Wu AY, et al. Analyses of antibacterial activity and cell compatibility of titanium coated with a Zr-C-N film. PLoS One. 2013; 8:e56771.
Article12. Oh EJ, Nguyen TDT, Lee SY, Jeon YM, Bae TS, Kim JG. Enhanced compatibility and initial stability of Ti6Al4V alloy orthodontic miniscrews subjected to anodization, cyclic precalcification, and heat treatment. Korean J Orthod. 2014; 44:246–253.
Article13. Cho YC, Cha JY, Hwang CJ, Park YC, Jung HS, Yu HS. Biologic stability of plasma ion-implanted miniscrews. Korean J Orthod. 2013; 43:120–126.
Article14. Crede CSF. Die verhutung der augenentzundung der neugeborenen (Ophthalmoblennorrhoea neonatorum) der haufigsten und wuchtigsten ursache der blindheit. Berlin: A. Hirschwald;1884.15. Buckley JJ, Lee AF, Olivic L, Wilsonb K. Hydroxyapatite supported antibacterial Ag3PO4 nanoparticles. J Mater Chem. 2010; 20:8056–8063.
Article16. Ciobanu CS, Massuyeau F, Constantin LV, Predoi D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100℃. Nanoscale Res Lett. 2011; 6:613.17. Hotta M, Nakajima H, Yamamoto K, Aono M. Antibacterial temporary filling materials: the effect of adding various ratios of Ag-Zn-Zeolite. J Oral Rehabil. 1998; 25:485–489.
Article18. Kvítek L, Panáćek A, Soukupová J, Kolář M, Večeřová R, Prucek R, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C. 2008; 112:5825–5834.
Article19. Rusu VM, Ng CH, Wilke M, Tiersch B, Fratzl P, Peter MG. Size-controlled hydroxyapatite nanoparticles as self-organized organic-inorganic composite materials. Biomaterials. 2005; 26:5414–5426.
Article20. Lim SI, Zhong CJ. Molecularly mediated processing and assembly of nanoparticles: exploring the interparticle interactions and structures. Acc Chem Res. 2009; 42:798–808.
Article21. Zheng J, Yu H, Li X, Zhang S. Enhanced photocatalytic activity of TiO2 nano-structured thin film with a silver hierarchical configuration. Appl Surf Sci. 2008; 254:1630–1635.
Article22. Díaz M, Barba F, Miranda M, Guitián F, Torrecillas R, Moya JS. Synthesis and antimicrobial activity of a silver-hydroxyapatite nanocomposite. J Nanomater. 2009; ID498505.
Article23. Moulder JF, Stickle WF, Sobol PE, Bomben KD. Handbook of X-ray photoelectron spectroscopy. Eden Prairie, MN: Perkin-Elmer Corp;1992.24. McQuillan JS, Infante HG, Stokes E, Shaw AM. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology. 2012; 6:857–866.
Article25. Suresh AK, Pelletier DA, Wang W, Moon JW, Gu B, Mortensen NP, et al. Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ Sci Technol. 2010; 44:5210–5215.
Article26. Xu H, Qu F, Xu H, Lai W, Andrew Wang Y, Aguilar ZP, et al. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157:H7. Biometals. 2012; 25:45–53.
Article27. Taheri S, Vasilev K, Majewski P. Silver nanoparticles: synthesis, antimicrobial coatings, and applications for medical devices. Recent Pat Mater Sci. 2015; 8:166–175.
Article28. Ritz HL. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967; 12:1561–1568.
Article29. Sambhy V, MacBride MM, Peterson BR, Sen A. Silver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materials. J Am Chem Soc. 2006; 128:9798–9808.
Article30. Shi Z, Neoh KG, Kang ET. Surface-grafted viologen for precipitation of silver nanoparticles and their combined bactericidal activities. Langmuir. 2004; 20:6847–6852.
Article31. Yuan W, Fu J, Su K, Ji J. Self-assembled chitosan/heparin multilayer film as a novel template for in situ synthesis of silver nanoparticles. Colloids Surf B Biointerfaces. 2010; 76:549–555.
Article32. Lischer S, Körner E, Balazs DJ, Shen D, Wick P, Grieder K, et al. Antibacterial burst-release from minimal Ag-containing plasma polymer coatings. J R Soc Interface. 2011; 8:1019–1030.
Article33. Ho CH, Tobis J, Sprich C, Thomann R, Tiller JC. Nanoseparated polymeric networks with multiple antimicrobial properties. Adv Mater. 2004; 16:957–961.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Silver Nanoparticles as a Smart Antimicrobial Agent
- In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles
- Adhesion of biofilm, surface characteristics, and mechanical properties of antimicrobial denture base resin
- Evaluation of the cell viability and antimicrobial effects of orthodontic bands coated with silver or zinc oxide nanoparticles: An in vitro study
- Silver nanoparticles in endodontics: recent developments and applications