Korean J Orthod.
2023 Jan;53(1):16-25. 10.4041/kjod22.091.
Evaluation of the cell viability and antimicrobial effects of orthodontic bands coated with silver or zinc oxide nanoparticles: An in vitro study
- Affiliations
-
- 1Department of Orthodontics, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
- 2Dental Research Center, Dentistry Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 3School of Chemistry, College of Science, University of Tehran, Tehran, Iran
- 4Department of Dental Biomaterials, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
- KMID: 2538710
- DOI: http://doi.org/10.4041/kjod22.091
Abstract
Objective
We aimed to evaluate the cell viability and antimicrobial effects of orthodontic bands coated with silver or zinc oxide nanoparticles (nanoAg and nano-ZnO, respectively).
Methods
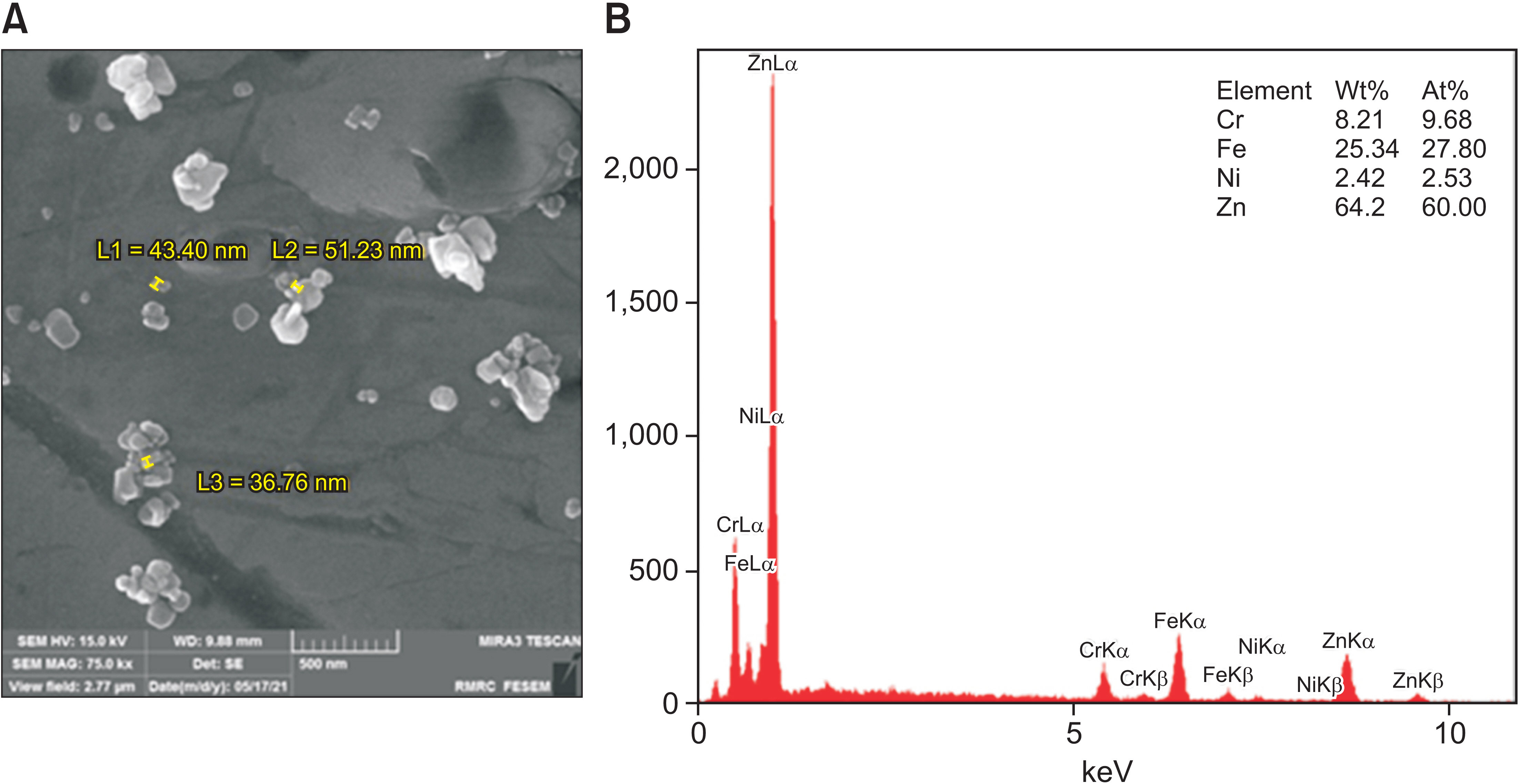
In this experimental study, 30 orthodontic bands were divided into three groups (n = 10 each): control (uncoated band), Ag (silver-coated band), and ZnO (zinc oxide-coated band). The electrostatic spray-assisted vapor deposition method was used to coat orthodontic bands with nano-Ag or nano-ZnO. The biofilm inhibition test was used to assess the antimicrobial effectiveness of nano-Ag and nano-ZnO against Streptococcus mutans, Lactobacillus acidophilus, and Candida albicans. Biocompatibility tests were conducted using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay. The groups were compared using oneway analysis of variance with a post-hoc test.
Results
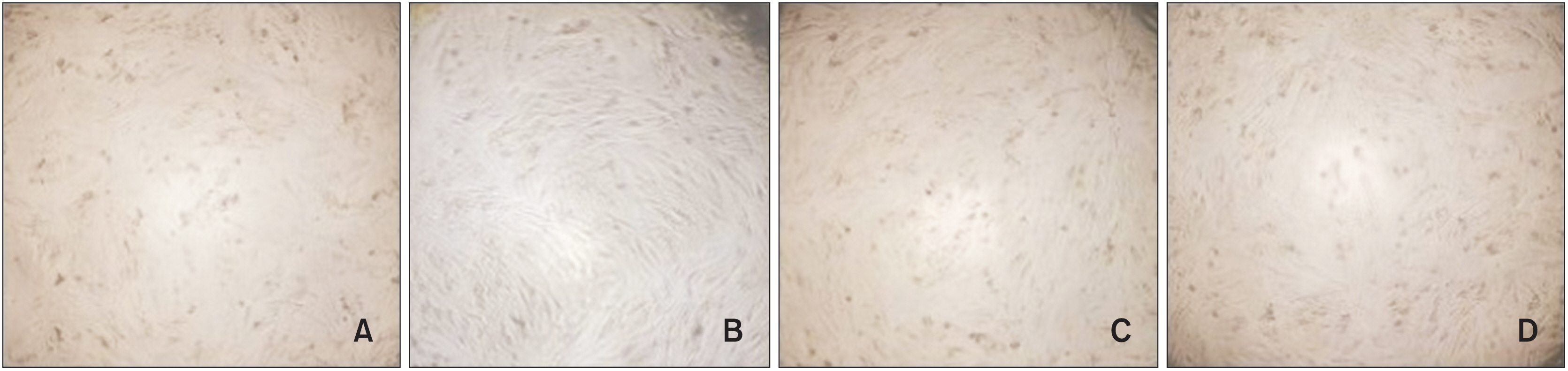
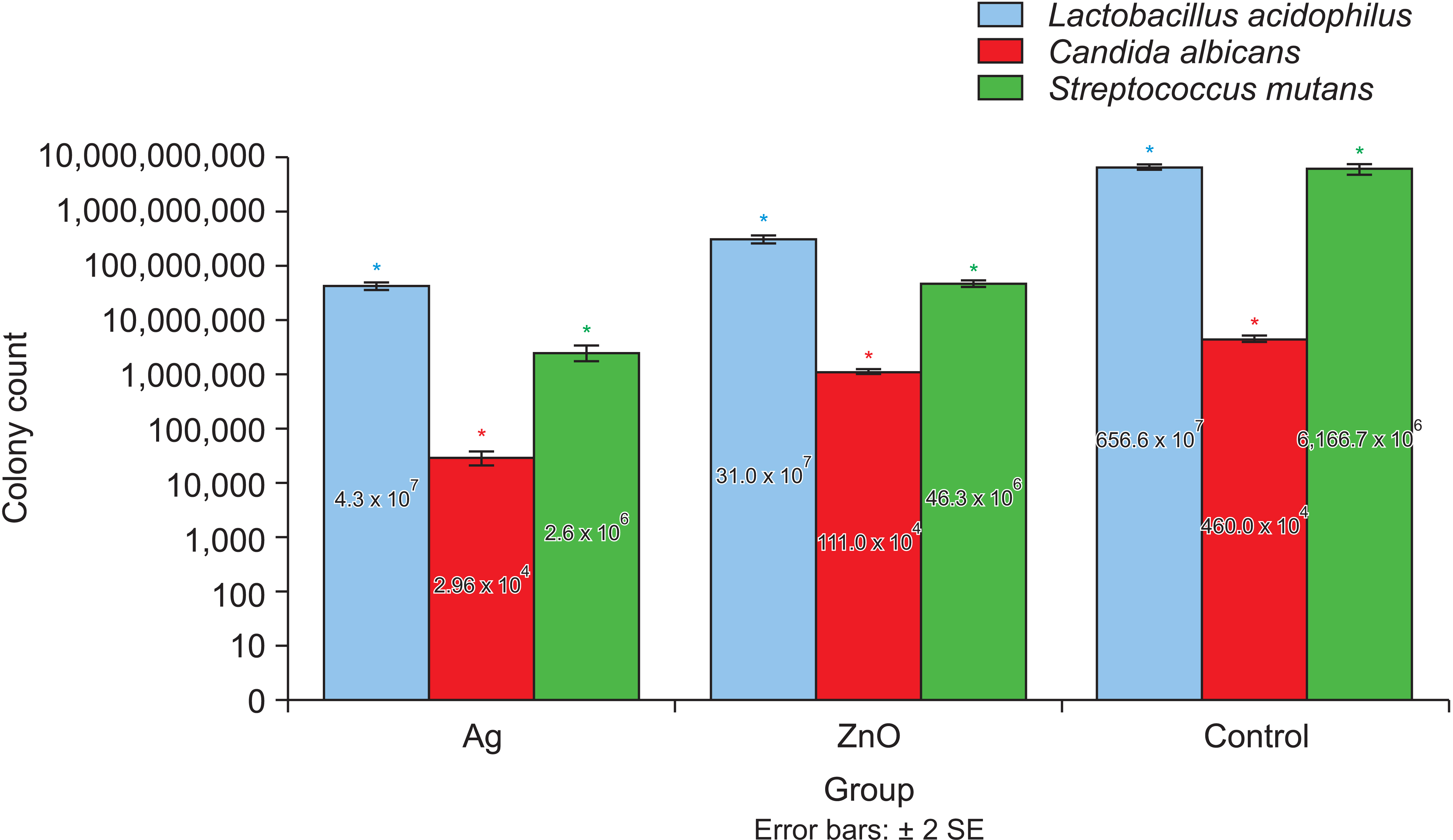
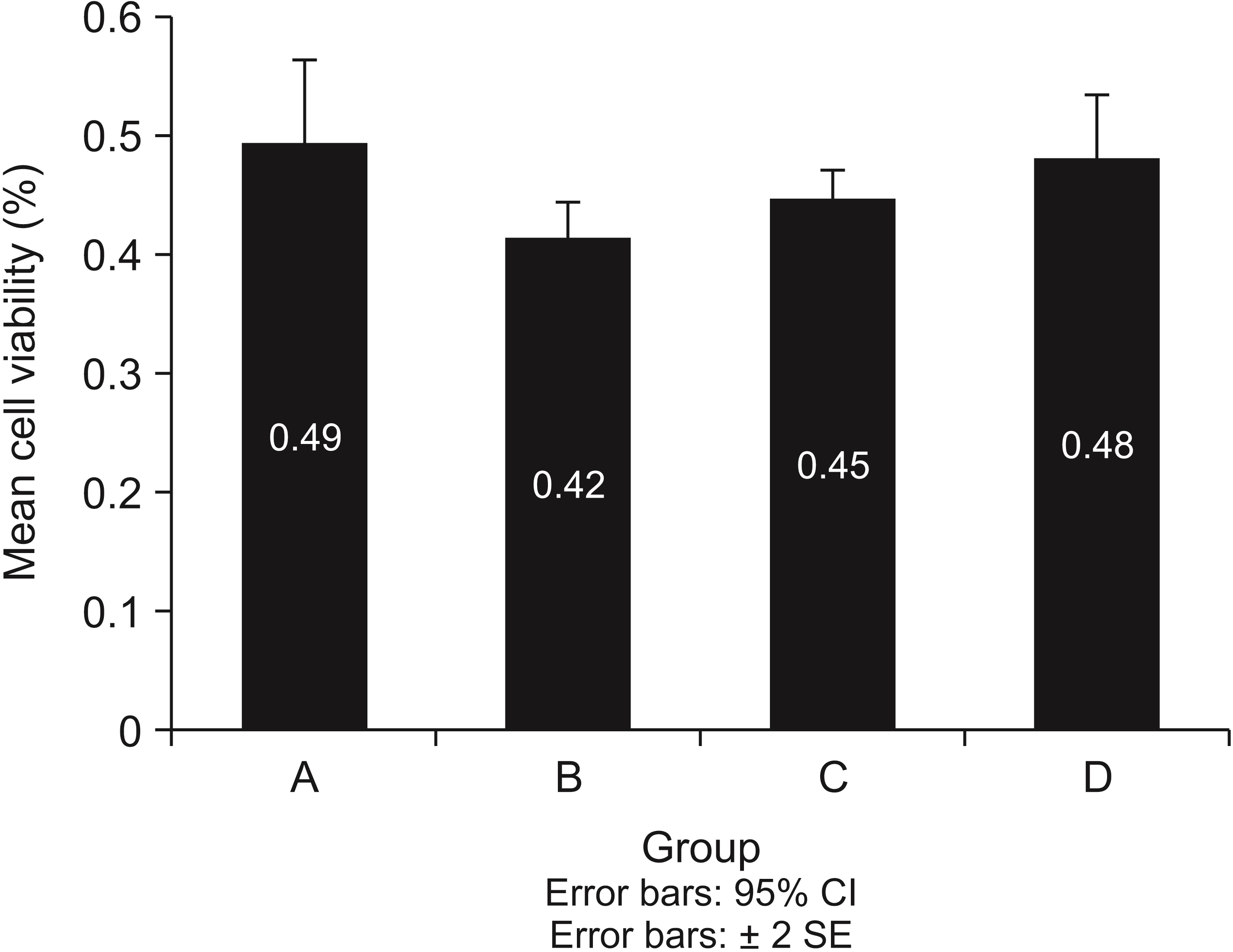
The Ag group showed a significantly higher reduction in the number of L. acidophilus, C. albicans, and S.mutans colonies than the ZnO group (p = 0.015, 0.003, and 0.005, respectively). Compared with the control group, the Ag group showed a 2-log 10 reduction in all the microorganisms' replication ability, but only S. mutants showed a 2-log10 reduction in replication ability in the ZnO group. The lowest mean cell viability was observed in the Ag group, but the difference between the groups was insignificant (p > 0.05).
Conclusions
Coating orthodontic bands with nanoZnO or nano-Ag induced antimicrobial effects against oral pathogens. Among the nanoparticles, nano-Ag showed the best antimicrobial activity and nanoZnO showed the highest biocompatibility.
Figure
Reference
-
1. Antonio-Zancajo L, Montero J, Albaladejo A, Oteo-Calatayud MD, Alvarado-Lorenzo A. 2020; Pain and oral-health-related quality of life in orthodontic patients during initial therapy with conventional, low-friction, and lingual brackets and aligners (Invisalign): a prospective clinical study. J Clin Med. 9:2088. DOI: 10.3390/jcm9072088. PMID: 32635196. PMCID: PMC7408790. PMID: ae922d85ec61459999b0394a5d12238d.
Article2. Erbe C, Hornikel S, Schmidtmann I, Wehrbein H. 2011; Quantity and distribution of plaque in orthodontic patients treated with molar bands. J Orofac Orthop. 72:13–20. DOI: 10.1007/s00056-010-0001-4. PMID: 21484542.
Article3. Anhoury P, Nathanson D, Hughes CV, Socransky S, Feres M, Chou LL. 2002; Microbial profile on metallic and ceramic bracket materials. Angle Orthod. 72:338–43. DOI: 10.1043/0003-3219(2002)072<0338:MPOMAC>2.0.CO;2. PMID: 12169034.4. Manuelli M, Marcolina M, Nardi N, Bertossi D, De Santis D, Ricciardi G, et al. 2019; Oral mucosal complications in orthodontic treatment. Minerva Stomatol. 68:84–8. DOI: 10.23736/S0026-4970.18.04127-4. PMID: 30854838.
Article5. Bishara SE, Ostby AW. 2008; White spot lesions: formation, prevention, and treatment. Semin Orthod. 14:174–82. DOI: 10.1053/j.sodo.2008.03.002.
Article6. Walsh LJ, Healey DL. 2019; Prevention and caries risk management in teenage and orthodontic patients. Aust Dent J. 64 Suppl 1:S37–45. DOI: 10.1111/adj.12671. PMID: 31144319.
Article7. Lacerda Rangel Esper MÂ, Junqueira JC, Uchoa AF, Bresciani E, Navarro RS, Nara de Souza Rastelli A, et al. 2019; Photodynamic inactivation of planktonic cultures and Streptococcus mutans biofilms for prevention of white spot lesions during orthodontic treatment: an in vitro investigation. Am J Orthod Dentofacial Orthop. 155:243–53. Erratum in: Am J Orthod Dentofacial Orthop 2019;155:458. DOI: 10.1016/j.ajodo.2018.03.027. PMID: 30712696.8. Beyth N, Houri-Haddad Y, Baraness-Hadar L, Yudovin-Farber I, Domb AJ, Weiss EI. 2008; Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 29:4157–63. DOI: 10.1016/j.biomaterials.2008.07.003. PMID: 18678404.
Article9. Lim BS, Lee SJ, Lee JW, Ahn SJ. 2008; Quantitative analysis of adhesion of cariogenic streptococci to orthodontic raw materials. Am J Orthod Dentofacial Orthop. 133:882–8. DOI: 10.1016/j.ajodo.2006.07.027. PMID: 18538253.
Article10. Shah AG, Shetty PC, Ramachandra CS, Bhat NS, Laxmikanth SM. 2011; In vitro assessment of photocatalytic titanium oxide surface modified stainless steel orthodontic brackets for antiadherent and antibacterial properties against Lactobacillus acidophilus. Angle Orthod. 81:1028–35. DOI: 10.2319/021111-101.1. PMID: 22007663. PMCID: PMC8903869.11. De Stefani A, Bruno G, Preo G, Gracco A. 2020; Application of nanotechnology in orthodontic materials: a state-of-the-art review. Dent J (Basel). 8:126. DOI: 10.3390/dj8040126. PMID: 33182424. PMCID: PMC7712537. PMID: edbfccbb4482466e9c6ea6eba21d3ea4.
Article12. Doudi M, Naghsh N, Heiedarpour A. 2011; The effect of silver nanoparticles on gram-negative bacilli resistant to extended-spectrum β-lactamase enzymes. Med Lab J. 5:44–51.13. Lloyd JR. 2003; Microbial reduction of metals and radionuclides. FEMS Microbiol Rev. 27:411–25. DOI: 10.1016/S0168-6445(03)00044-5. PMID: 12829277.
Article14. Bürgers R, Eidt A, Frankenberger R, Rosentritt M, Schweikl H, Handel G, et al. 2009; The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch Oral Biol. 54:595–601. DOI: 10.1016/j.archoralbio.2009.03.004. PMID: 19375069.
Article15. Spacciapoli P, Buxton D, Rothstein D, Friden P. 2001; Antimicrobial activity of silver nitrate against periodontal pathogens. J Periodontal Res. 36:108–13. DOI: 10.1034/j.1600-0765.2001.360207.x. PMID: 11327077.
Article16. Ohira T, Yamamoto O, Iida Y, Nakagawa ZE. 2008; Antibacterial activity of ZnO powder with crystallographic orientation. J Mater Sci Mater Med. 19:1407–12. DOI: 10.1007/s10856-007-3246-8. PMID: 17914627.
Article17. Afonso Camargo SE, Mohiuddeen AS, Fares C, Partain JL, Carey PH 4th, Ren F, et al. 2020; Anti-bacterial properties and biocompatibility of novel SiC coating for dental ceramic. J Funct Biomater. 11:33. DOI: 10.3390/jfb11020033. PMID: 32443691. PMCID: PMC7353563. PMID: 114fa56d7f834778a4ccad9da19491fe.
Article18. Miles AA, Misra SS, Irwin JO. 1938; The estimation of the bactericidal power of the blood. J Hyg (Lond). 38:732–49. DOI: 10.1017/S002217240001158X. PMID: 20475467. PMCID: PMC2199673.
Article19. International Organization for Standardization (ISO). 2009. ISO 10993-5:2009. Biological evaluation of medical devices- part 5: tests for in vitro cytotoxicity. ISO Copyright Office;Geneva:20. Tavassoli-Hojjati S, Haghgoo R, Mehran M, Niktash A. 2012; Evaluation of the effect of fluoride gel and varnish on the demineralization resistance of enamel: an in vitro. J Iran Dent Assoc. 24:28–34.21. Farhadian N, Usefi Mashoof R, Khanizadeh S, Ghaderi E, Farhadian M, Miresmaeili A. 2016; Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs those wearing conventional retainers: a randomized clinical trial. Am J Orthod Dentofacial Orthop. 149:155–60. Erratum in: Am J Orthod Dentofacial Orthop 2017;151:11. DOI: 10.1016/j.ajodo.2015.07.031. PMID: 26827971.22. Arash V, Keikhaee F, Rabiee SM, Rajabnia R, Khafri S, Tavanafar S. 2016; Evaluation of antibacterial effects of silver-coated stainless steel orthodontic brackets. J Dent (Tehran). 13:49–54. PMID: 27536328. PMCID: PMC4983565. PMID: 93db76e397d94eb4b16f3f304c3d33e2.23. Ghorbanzadeh R, Pourakbari B, Bahador A. 2015; Effects of baseplates of orthodontic appliances with in situ generated silver nanoparticles on cariogenic bacteria: a randomized, double-blind cross-over clinical trial. J Contemp Dent Pract. 16:291–8. DOI: 10.5005/jp-journals-10024-1678. PMID: 26067732.
Article24. Poosti M, Ramazanzadeh B, Zebarjad M, Javadzadeh P, Naderinasab M, Shakeri MT. 2013; Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur J Orthod. 35:676–9. DOI: 10.1093/ejo/cjs073. PMID: 23264617.
Article25. Jedrychowski JR, Caputo AA, Kerper S. 1983; Antibacterial and mechanical properties of restorative materials combined with chlorhexidines. J Oral Rehabil. 10:373–81. DOI: 10.1111/j.1365-2842.1983.tb00133.x. PMID: 6355413.
Article26. Bulut H, Türkün M, Türkün LS, Işiksal E. 2007; Evaluation of the shear bond strength of 3 curing bracket bonding systems combined with an antibacterial adhesive. Am J Orthod Dentofacial Orthop. 132:77–83. DOI: 10.1016/j.ajodo.2005.06.040. PMID: 17628254.
Article27. Kachoei M, Divband B, Rahbar M, Esmaeilzadeh M, Ghanizadeh M, Alam M. 2021; A novel developed bioactive composite resin containing silver/zinc oxide (Ag/ZnO) nanoparticles as an antimicrobial material against Streptococcus mutans, Lactobacillus, and Candida albicans. Evid Based Complement Alternat Med. 2021:4743411. DOI: 10.1155/2021/4743411. PMID: 34697547. PMCID: PMC8541865.28. Garmasheva I, Kovalenko N, Voychuk S, Ostapchuk A, Livins'ka O, Oleschenko L. 2016; Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens in vitro. Bioimpacts. 6:219–23. DOI: 10.15171/bi.2016.29. PMID: 28265538. PMCID: PMC5326670.
Article29. Sharma VK, Yngard RA, Lin Y. 2009; Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 145:83–96. DOI: 10.1016/j.cis.2008.09.002. PMID: 18945421.
Article30. Chen X, Schluesener HJ. 2008; Nanosilver: a nanoproduct in medical application. Toxicol Lett. 176:1–12. DOI: 10.1016/j.toxlet.2007.10.004. PMID: 18022772.
Article31. Hernández-Sierra JF, Ruiz F, Pena DC, Martínez-Gutiérrez F, Martínez AE, Guillén Ade J, et al. 2008; The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 4:237–40. DOI: 10.1016/j.nano.2008.04.005. PMID: 18565800.
Article32. Cieplik F, Aparicio C, Kreth J, Schmalz G. 2022; Development of standard protocols for biofilm-biomaterial interface testing. JADA Found Sci. 1:100008. DOI: 10.1016/j.jfscie.2022.100008.
Article33. Kasraei S, Sami L, Hendi S, Alikhani MY, Rezaei-Soufi L, Khamverdi Z. 2014; Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod. 39:109–14. DOI: 10.5395/rde.2014.39.2.109. PMID: 24790923. PMCID: PMC3978100.
Article34. Hailan SY, Al-Khatieeb MM. 2019; Antimicrobial efficacy of silver, zinc oxide, and titanium dioxide nanoparticles incorporated in orthodontic bonding agent. J Baghdad Coll Dent. 31:10–6. DOI: 10.26477/jbcd.v31i3.2693. PMID: 2adb939a2c1747d28c8cc28b100e6b21.
Article35. Ahrari F, Eslami N, Rajabi O, Ghazvini K, Barati S. 2015; The antimicrobial sensitivity of Streptococcus mutans and Streptococcus sangius to colloidal solutions of different nanoparticles applied as mouthwashes. Dent Res J (Isfahan). 12:44–9. DOI: 10.4103/1735-3327.150330. PMID: 25709674. PMCID: PMC4336971.
Article36. Prabha RD, Kandasamy R, Sivaraman US, Nandkumar MA, Nair PD. 2016; Antibacterial nanosilver coated orthodontic bands with potential implications in dentistry. Indian J Med Res. 144:580–6. DOI: 10.4103/0971-5916.200895. PMID: 28256467. PMCID: PMC5345305.37. Halimi SU, Bakar NFA, Ismail SN, Hashib SA. 2014; Electrospray deposition of titanium dioxide (TiO2) nanoparticles. AIP Conf Proc. 1586:57. DOI: 10.1063/1.4866730.
Article38. Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. 2013; Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol. 87:1181–200. DOI: 10.1007/s00204-013-1079-4. PMID: 23728526. PMCID: PMC3677982.
Article39. Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J, et al. 2009; Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro. 23:1076–84. DOI: 10.1016/j.tiv.2009.06.001. PMID: 19508889.
Article40. Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C. 2013; Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc. 135:1438–44. DOI: 10.1021/ja309812z. PMID: 23301582.
Article41. Shahi S, Özcan M, Maleki Dizaj S, Sharifi S, Al-Haj Husain N, Eftekhari A, et al. 2019; A review on potential toxicity of dental material and screening their biocompatibility. Toxicol Mech Methods. 29:368–77. DOI: 10.1080/15376516.2019.1566424. PMID: 30642212.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Skin corrosion and irritation test of sunscreen nanoparticles using reconstructed 3D human skin model
- Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus
- Silver Nanoparticles as a Smart Antimicrobial Agent
- In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles
- An experimental study on the cytotoxicity of various orthodontic bands