J Periodontal Implant Sci.
2013 Apr;43(2):72-78.
Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens
- Affiliations
-
- 1Department of Periodontology, Research Institute for Oral Science, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea. dentist@gwnu.ac.kr
- 2Department of Microbiology and Immunology, Research Institute for Oral Science, Gangneung-Wonju National University College of Dentistry, Gangneung, Korea.
- 3Department of Periodontics, Kangbuk Samsung Hospital, Seoul, Korea.
Abstract
- PURPOSE
The purpose of this study was to compare the phototoxic effects of blue light exposure on periodontal pathogens in both planktonic and biofilm cultures.
METHODS
Strains of Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, and Porphyromonas gingivalis, in planktonic or biofilm states, were exposed to visible light at wavelengths of 400.520 nm. A quartz-tungsten-halogen lamp at a power density of 500 mW/cm2 was used for the light source. Each sample was exposed to 15, 30, 60, 90, or 120 seconds of each bacterial strain in the planktonic or biofilm state. Confocal scanning laser microscopy (CSLM) was used to observe the distribution of live/dead bacterial cells in biofilms. After light exposure, the bacterial killing rates were calculated from colony forming unit (CFU) counts.
RESULTS
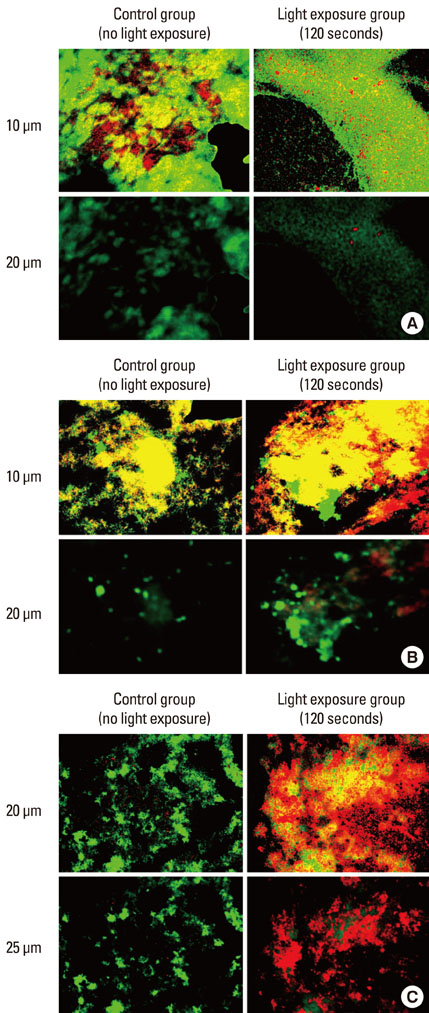
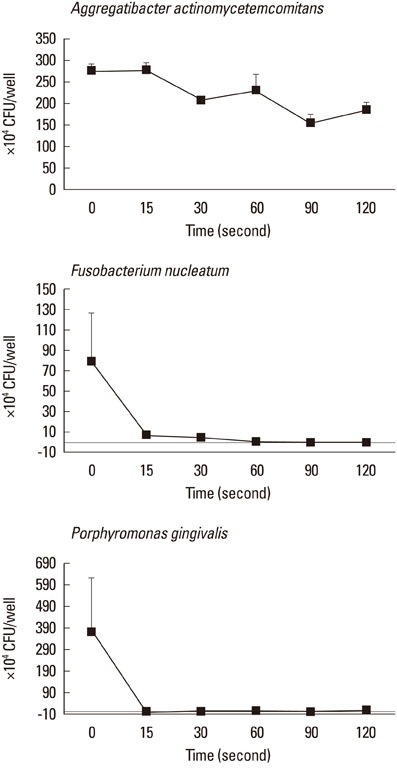
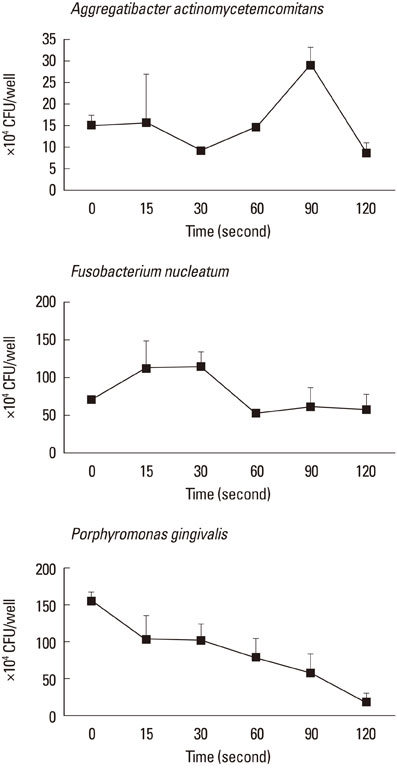
CLSM images that were obtained from biofilms showed a mixture of dead and live bacterial cells extending to a depth of 30-45 microm. Obvious differences in the live-to-dead bacterial cell ratio were found in P. gingivalis biofilm according to light exposure time. In the planktonic state, almost all bacteria were killed with 60 seconds of light exposure to F. nucleatum (99.1%) and with 15 seconds to P. gingivalis (100%). In the biofilm state, however, only the CFU of P. gingivalis demonstrated a decreasing tendency with increasing light exposure time, and there was a lower efficacy of phototoxicity to P. gingivalis as biofilm than in the planktonic state.
CONCLUSIONS
Blue light exposure using a dental halogen curing unit is effective in reducing periodontal pathogens in the planktonic state. It is recommended that an adjunctive exogenous photosensitizer be used and that pathogens be exposed to visible light for clinical antimicrobial periodontal therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol 2000. 1997. 14:12–32.
Article2. Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res. 2009. 44:751–759.
Article3. Adriaens PA, Adriaens LM. Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontol 2000. 2004. 36:121–145.
Article4. Umeda M, Takeuchi Y, Noguchi K, Huang Y, Koshy G, Ishikawa I. Effects of nonsurgical periodontal therapy on the microbiota. Periodontol 2000. 2004. 36:98–120.
Article5. Amano A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front Biosci. 2007. 12:3965–3974.
Article6. Meyer DH, Sreenivasan PK, Fives-Taylor PM. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991. 59:2719–2726.
Article7. Thiha K, Takeuchi Y, Umeda M, Huang Y, Ohnishi M, Ishikawa I. Identification of periodontopathic bacteria in gingival tissue of Japanese periodontitis patients. Oral Microbiol Immunol. 2007. 22:201–207.
Article8. Anderson GG, O'Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008. 322:85–105.
Article9. del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007. 82:204–209.
Article10. Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005. 13:34–40.
Article11. Wilson M. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci. 2004. 3:412–418.
Article12. Takasaki AA, Aoki A, Mizutani K, Schwarz F, Sculean A, Wang CY, et al. Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000. 2009. 51:109–140.
Article13. Maisch T. Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci. 2007. 22:83–91.
Article14. Maisch T, Szeimies RM, Jori G, Abels C. Antibacterial photodynamic therapy in dermatology. Photochem Photobiol Sci. 2004. 3:907–917.
Article15. Sharman WM, Allen CM, van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today. 1999. 4:507–517.
Article16. Duerden BI. Pigment production by Bacteroides species with reference to sub-classification. J Med Microbiol. 1975. 8:113–125.
Article17. Reid JS, Beeley JA, MacFarlane TW. A study of the pigment produced by Bacteroides melaninogenicus. J Dent Res. 1976. 55:1130.
Article18. Shah HN, Bonnett R, Mateen B, Williams RA. The porphyrin pigmentation of subspecies of Bacteroides melaninogenicus. Biochem J. 1979. 180:45–50.
Article19. Loesche WJ. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969. 18:723–727.
Article20. Loesche WJ, Gusberti F, Mettraux G, Higgins T, Syed S. Relationship between oxygen tension and subgingival bacterial flora in untreated human periodontal pockets. Infect Immun. 1983. 42:659–667.
Article21. Henry CA, Dyer B, Wagner M, Judy M, Matthews JL. Phototoxicity of argon laser irradiation on biofilms of Porphyromonas and Prevotella species. J Photochem Photobiol B. 1996. 34:123–128.
Article22. Henry CA, Judy M, Dyer B, Wagner M, Matthews JL. Sensitivity of Porphyromonas and Prevotella species in liquid media to argon laser. Photochem Photobiol. 1995. 61:410–413.
Article23. Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss EI. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem Photobiol. 2005. 81:1186–1189.
Article24. Feuerstein O, Persman N, Weiss EI. Phototoxic effect of visible light on Porphyromonas gingivalis and Fusobacterium nucleatum: an in vitro study. Photochem Photobiol. 2004. 80:412–415.
Article25. Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, et al. Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother. 2005. 49:1391–1396.
Article26. Okamoto K, Nakayama K, Kadowaki T, Abe N, Ratnayake DB, Yamamoto K. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J Biol Chem. 1998. 273:21225–21231.
Article27. Smalley JW, Silver J, Marsh PJ, Birss AJ. The periodontopathogen Porphyromonas gingivalis binds iron protoporphyrin IX in the mu-oxo dimeric form: an oxidative buffer and possible pathogenic mechanism. Biochem J. 1998. 331(Pt 3):681–685.
Article28. Redmond RW, Gamlin JN. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem Photobiol. 1999. 70:391–475.
Article29. Gourmelon M, Cillard J, Pommepuy M. Visible light damage to Escherichia coli in seawater: oxidative stress hypothesis. J Appl Bacteriol. 1994. 77:105–112.
Article30. Webb RB, Malina MM. Mutagenesis in Escherichia coli by visible light. Science. 1967. 156:1104–1105.31. Müller P, Guggenheim B, Schmidlin PR. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm in vitro. Eur J Oral Sci. 2007. 115:77–80.
Article32. Soukos NS, Socransky SS, Mulholland SE, Lee S, Doukas AG. Photomechanical drug delivery into bacterial biofilms. Pharm Res. 2000. 17:405–409.33. Dahl TA, Midden WR, Hartman PE. Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen. J Bacteriol. 1989. 171:2188–2194.
Article34. Werner E, Roe F, Bugnicourt A, Franklin MJ, Heydorn A, Molin S, et al. Stratified growth in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2004. 70:6188–6196.
Article35. Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001. 35:439–468.
Article36. Hoiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen PO, et al. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011. 3:55–65.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Susceptibility of Mutans streptococci in the Planktonic and Biofilm State to Erythrosine
- Inhibition of biofilm formation of periodontal pathogens by D-Arabinose
- Application of Teeth Whitening LED for Prevention of Dental Caries : Antimicrobial Photodynamic Therapy Approach
- Antimicrobial Effect on Streptococcus mutans in Photodynamic Therapy using Different Light Source
- Quorum Sensing Regulation of Biofilm Formation by Periodontal Pathogens