World J Mens Health.
2013 Aug;31(2):150-156.
The Anti-Inflammatory Effects of a New Herbal Formula (WSY-1075) in a Nonbacterial Prostatitis Rat Model
- Affiliations
-
- 1Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. ksw1227@catholic.ac.kr
- 2Korea Bio Medical Science Institute, Seoul, Korea.
Abstract
- PURPOSE
The aim of this study was to investigate the anti-inflammatory effects of a new herbal formula (WSY-1075) in a nonbacterial prostatitis rat model.
MATERIALS AND METHODS
Prostatitis was induced in male Wistar rats (n=32) by treatment with 17 beta-estradiol and dihydrotestosterone for 4 weeks. After the induction of prostatitis, the rats were randomly divided into one of four treatment groups: control (n=8), ciprofloxacin (n=8), WSY-1075 (100 mg/kg) (n=8), and WSY-1075 (400 mg/kg) (n=8). After 4 weeks of treatment, the prostatic proinflammatory cytokine (tumor necrosis factor-alpha, interleukin [IL]-6, and IL-8) levels and histological findings were noted.
RESULTS
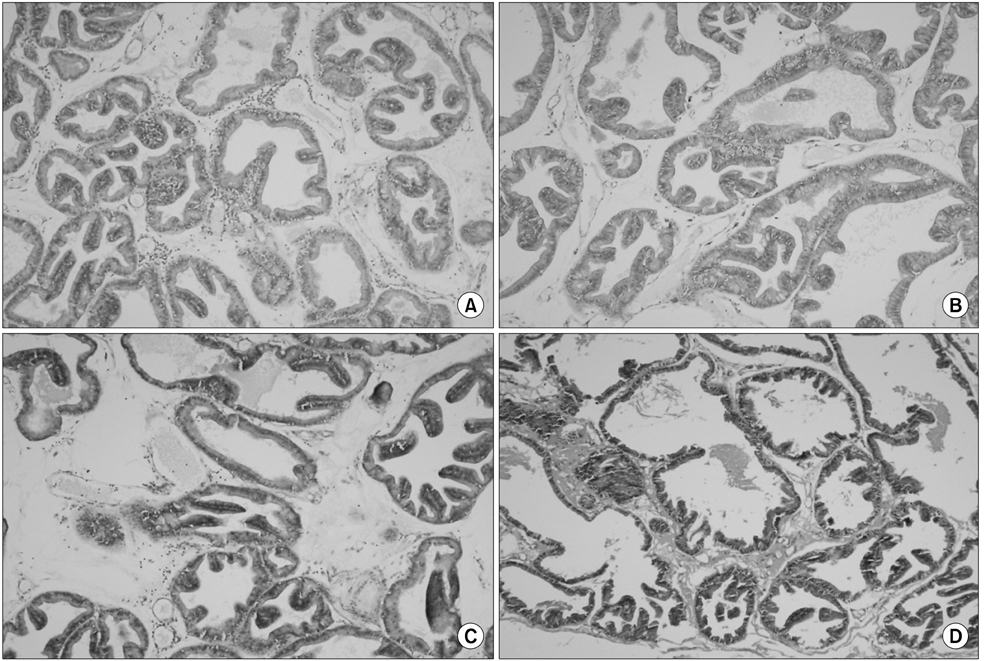
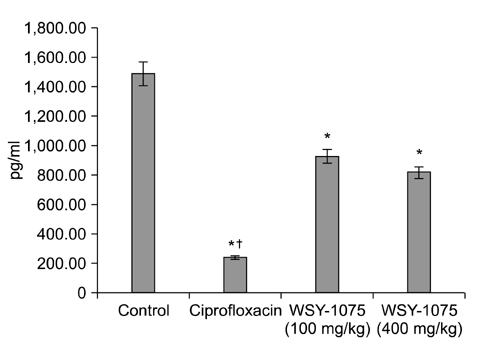
The ciprofloxacin and WSY-1075 treatment groups showed significantly decreased proinflammatory cytokine levels compared with the control group. Histologically, treatment with ciprofloxacin and WSY-1075 significantly suppressed the severity of prostatitis lesions compared with those in the control group. No differences in the proinflammatory cytokine levels or histologic findings were observed with the dose dependent treatment of WSY-1075.
CONCLUSIONS
The new herbal formula, WSY-1075, showed effective anti-inflammatory activities in the prostate and may be useful for the clinical treatment of nonbacterial prostatitis. Our findings suggest that WSY-1075 has a beneficial effect on the prevention and treatment of nonbacterial prostatitis.
Keyword
MeSH Terms
Figure
Reference
-
1. Fowler JE Jr. Prostatitis. In : Gillenwater JY, Grayhack JT, Howard SS, Duckett JW, editors. Adult and pediatric urology. 2nd ed. St. Louis: Mosby Year Book;1991. p. 1395–1423.2. Nadler RB, Koch AE, Calhoun EA, Campbell PL, Pruden DL, Bennett CL, et al. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000; 164:214–218.3. Penna G, Mondaini N, Amuchastegui S, Degli Innocenti S, Carini M, Giubilei G, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007; 51:524–533.
Article4. Vykhovanets EV, Resnick MI, MacLennan GT, Gupta S. Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis. 2007; 10:15–29.
Article5. Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988; 140:1049–1053.
Article6. Seethalakshmi L, Bala RS, Malhotra RK, Austin-Ritchie T, Miller-Graziano C, Menon M, et al. 17 beta-estradiol induced prostatitis in the rat is an autoimmune disease. J Urol. 1996; 156:1838–1842.
Article7. Lundgren R, Holmquist B, Hesselvik M, Müntzing J. Treatment of prostatitis in the rat. Prostate. 1984; 5:277–284.
Article8. Müntzing J, Sufrin G, Murphy GP. Prostatitis in the rat. Scand J Urol Nephrol. 1979; 13:17–22.
Article9. Shin S, Jeon JH, Park D, Jang JY, Joo SS, Hwang BY, et al. Anti-inflammatory effects of an ethanol extract of Angelica gigas in a Carrageenan-air pouch inflammation model. Exp Anim. 2009; 58:431–436.10. Park CH, Noh JS, Kim JH, Tanaka T, Zhao Q, Matsumoto K, et al. Evaluation of morroniside, iridoid glycoside from Corni Fructus, on diabetes-induced alterations such as oxidative stress, inflammation, and apoptosis in the liver of type 2 diabetic db/db mice. Biol Pharm Bull. 2011; 34:1559–1565.
Article11. Lee JH, Lee JH, Lee YM, Kim PN, Jeong CS. Potential analgesic and anti-inflammatory activities of Panax ginseng head butanolic fraction in animals. Food Chem Toxicol. 2008; 46:3749–3752.12. Oh YC, Cho WK, Im GY, Jeong YH, Hwang YH, Liang C, et al. Anti-inflammatory effect of Lycium Fruit water extract in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Int Immunopharmacol. 2012; 13:181–189.
Article13. Kim KW, Kim KS, Park SD, Kim JK, Chung KH, Kim DS, et al. Effect of Cervus korean TEMMINCK var. mantchuricus Swinhoe on protease activities, antioxidant and free radical damages in rheumatis arthritis rats. Toxicol In Vitro. 2008; 22:80–86.
Article14. Robinette CL. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate. 1988; 12:271–286.
Article15. Oka M, Tachibana M, Noda K, Inoue N, Tanaka M, Kuwabara K. Relevance of anti-reactive oxygen species activity to anti-inflammatory activity of components of eviprostat, a phytotherapeutic agent for benign prostatic hyperplasia. Phytomedicine. 2007; 14:465–472.
Article16. Liu L, Li Q, Han P, Li X, Zeng H, Zhu Y, et al. Evaluation of interleukin-8 in expressed prostatic secretion as a reliable biomarker of inflammation in benign prostatic hyperplasia. Urology. 2009; 74:340–344.
Article17. Bernoulli J, Yatkin E, Konkol Y, Talvitie EM, Santti R, Streng T. Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate. 2008; 68:1296–1306.
Article18. Domingue GJ Sr, Hellstrom WJ. Prostatitis. Clin Microbiol Rev. 1998; 11:604–613.
Article19. Arakawa S, Matsui T, Gohji K, Okada H, Kamidono S. Prostatitis--the Japanese viewpoint. Int J Antimicrob Agents. 1999; 11:201–203.
Article20. Donovan DA, Nicholas PK. Prostatitis: diagnosis and treatment in primary care. Nurse Pract. 1997; 22:144–146.21. Mishra VC, Allen DJ, Nicolaou C, Sharif H, Hudd C, Karim OM, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007; 100:327–331.
Article22. Roehrborn CG. Definition of at-risk patients: baseline variables. BJU Int. 2006; 97:Suppl 2. 7–11.
Article23. Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999; 84:976–981.
Article24. Kim J, Kim K. Inhibitory effects of cervi pantotrichum cornu herbal acupuncture on type II collagen-induced arthritis. J Korean Acupunct Moxibustion Soc. 2002; 19:155–170.25. Jung JS, Shin JA, Park EM, Lee JE, Kang YS, Min SW, et al. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J Neurochem. 2010; 115:1668–1680.
Article26. Sung YH, Chang HK, Kim SE, Kim YM, Seo JH, Shin MC, et al. Anti-inflammatory and analgesic effects of the aqueous extract of corni fructus in murine RAW 264.7 macrophage cells. J Med Food. 2009; 12:788–789.
Article27. Ahn KS, Sim WS, Lee IK, Seu YB, Kim IH. Decursinol angelate: a cytotoxic and protein kinase C activating agent from the root of Angelica gigas. Planta Med. 1997; 63:360–361.28. Bae EA, Han MJ, Kim NJ, Kim DH. Anti-Helicobacter pylori activity of herbal medicines. Biol Pharm Bull. 1998; 21:990–992.
Article29. Cho S, Takahashi M, Toita S, Cyong J. Suppression of adjuvant arthritis on rat by oriental herbs. Shoyakugaku zasshi. 1982; 36:78–81.30. Kim JH, Jeong JH, Jeon ST, Kim H, Ock J, Suk K, et al. Decursin inhibits induction of inflammatory mediators by blocking nuclear factor-kappaB activation in macrophages. Mol Pharmacol. 2006; 69:1783–1790.31. Zhang R, Kang KA, Piao MJ, Kim KC, Kim AD, Chae S, et al. Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J Ethnopharmacol. 2010; 130:299–306.32. Amagase H, Sun B, Nance DM. Immunomodulatory effects of a standardized Lycium barbarum fruit juice in Chinese older healthy human subjects. J Med Food. 2009; 12:1159–1165.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-Inflammatory and Antimicrobial Effects of a Novel Herbal Formulation (WSY-1075) in a Chronic Bacterial Prostatitis Rat Model
- Anti-Inflammatory Effect of Phlorotannin on Chronic Nonbacterial Prostatitis in a Rat Model
- Letter to the Editor regarding the Article: “Anti-Inflammatory and Antimicrobial Effects of a Novel Herbal Formulation (WSY-1075) in a Chronic Bacterial Prostatitis Rat Model†by Park et al. World J Mens Health 2016;34(3):179-185
- Improvement of Persistent Detrusor Overactivity through Treatment with a Phytotherapeutic Agent (WSY-1075) after Relief of Bladder Outlet Obstruction
- Sperm Motility in Chronic Nonbacterial Prostatitis: Related to Antisperm Antibody and Seminal Cytokine and Seminal Plasma Composition