Korean J Urogenit Tract Infect Inflamm.
2014 Oct;9(2):86-92. 10.14777/kjutii.2014.9.2.86.
Anti-Inflammatory Effect of Phlorotannin on Chronic Nonbacterial Prostatitis in a Rat Model
- Affiliations
-
- 1Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. urohan@catholic.ac.kr
- KMID: 1732620
- DOI: http://doi.org/10.14777/kjutii.2014.9.2.86
Abstract
- PURPOSE
Chronic nonbacterial prostatitis and chronic pelvic pain syndrome account for 90-95% of all prostatitis. Little is known about its pathophysiology, thus, various treatments are used. Ecklonia cava, a seaweed, is a member of the brown algae family; many recent reports have demonstrated that its extract containing phlorotannin has anti-oxidative and anti-inflammatory properties. Using the hormone-induced prostatitis rat model, we investigated the anti-inflammatory effects of E. cava extracts via its anti-oxidative process on chronic nonbacterial prostatitis.
MATERIALS AND METHODS
Forty, 10-week-old male white Wistar rats were utilized, and divided equally into the following five groups: 1) control, 2) E. cava-fed, 3) hormone-induced prostatitis (HIP), 4) E. cava-treated HIP, and 5) nonsteroidal anti-inflammatory drug (NSAID)-treated HIP.
RESULTS
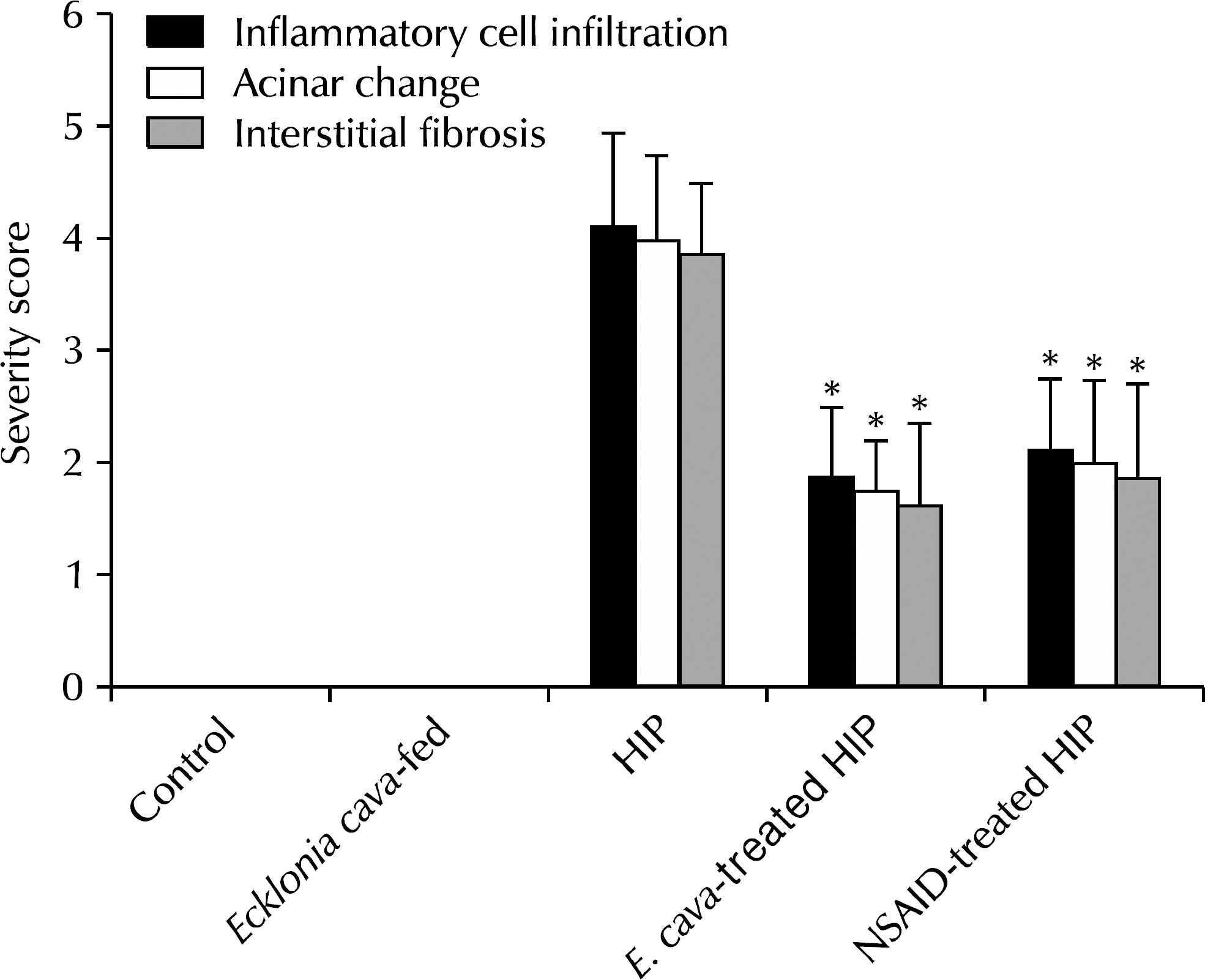
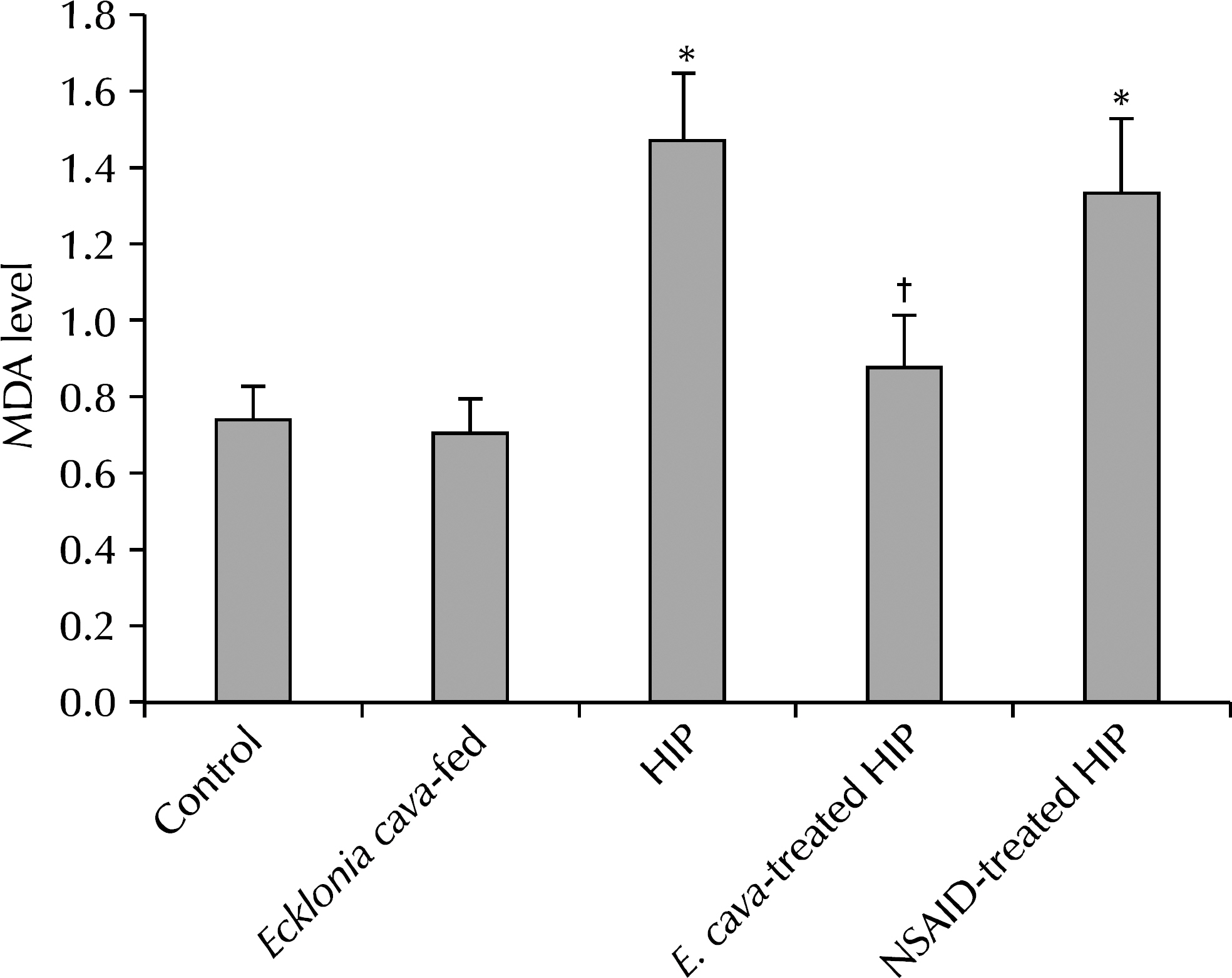
The results showed statistically-significant improvement in the tissue response to the hormone-induced inflammation among the E. cava-treated and NSAID-treated groups (p<0.05). Lower malonedialdehyde levels were observed in the group with E. cava-treated HIP than with HIP alone, which was statistically significant. We believe that this supports the anti-oxidative properties of E. cava.
CONCLUSIONS
This study demonstrates that phlorotannin has anti-inflammatory properties via its anti-oxidative process, which we expect to play an important role in prevention and as an adjuvant therapy for chronic nonbacterial prostatitis.
Keyword
MeSH Terms
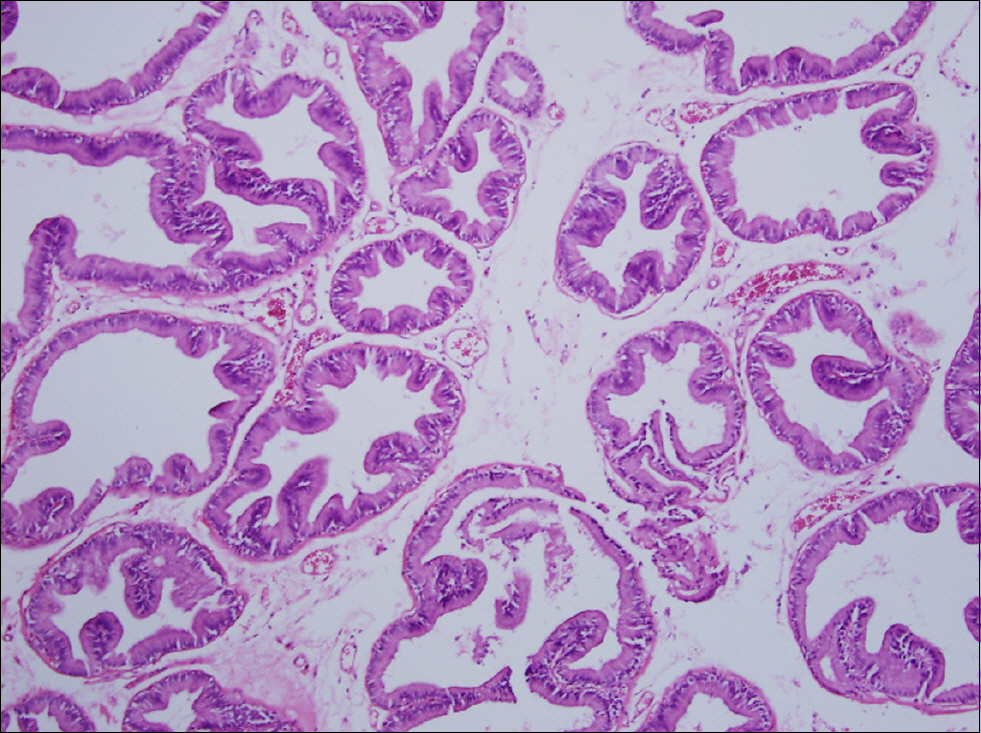
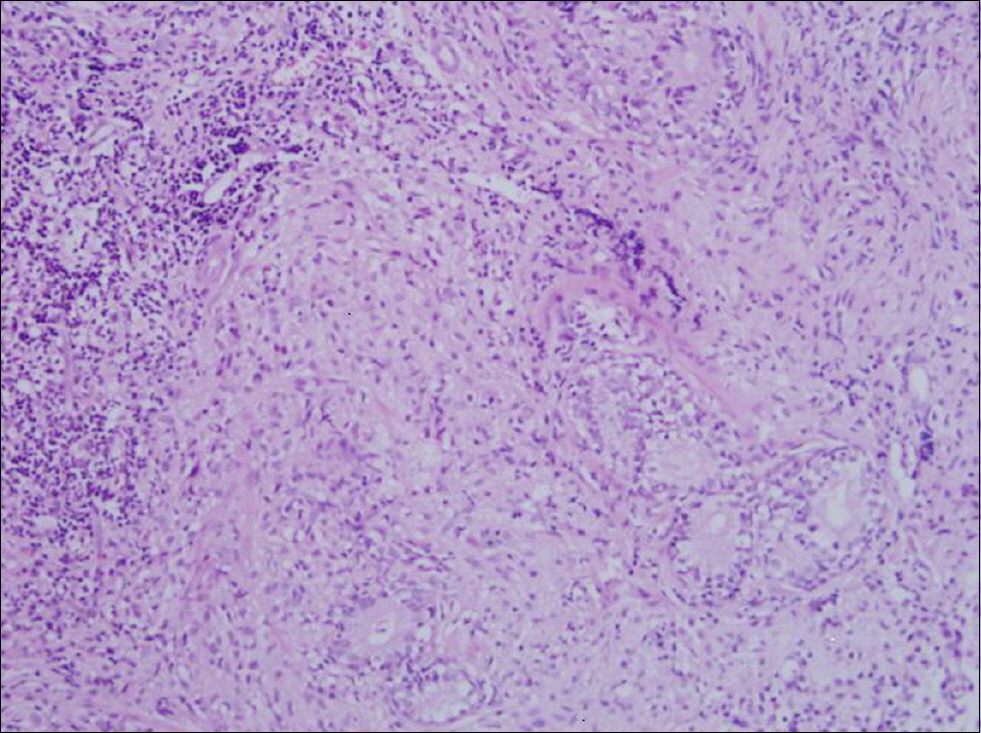
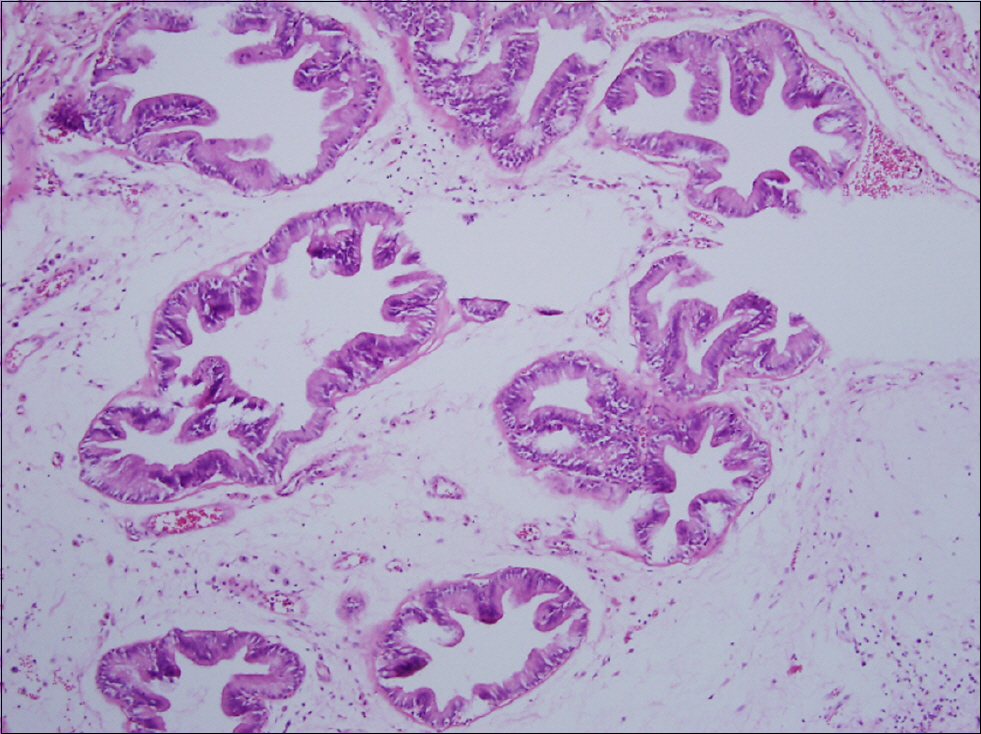
Figure
Reference
-
1. Nickel JC, Nyberg LM, Hennenfent M. Research guidelines for chronic prostatitis: consensus report from the first National Institutes of Health International Prostatitis Collaborative Network. Urology. 1999; 54:229–33.
Article2. Nickel JC, Downey J, Hunter D, Clark J. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. J Urol. 2001; 165:842–5.
Article3. Bahk JY, Hyun JS, Lee JY, Kim J, Cho YH, Lee JH, et al. Concentration of ofloxacin in canine prostate tissue and prostate fluid after intraprostatic injection of biodegradable sustained-releasing microspheres containing ofloxacin. J Urol. 2000; 163:1560–4.
Article4. Faulkner DJ. Marine natural products. Nat Prod Rep. 2002; 19:1–48.
Article5. Wijesinghe WA, Jeon YJ. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: a review. Int J Food Sci Nutr. 2012; 63:225–35.6. Robinette CL. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate. 1988; 12:271–86.
Article7. Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988; 140:1049–53.
Article8. Kim DS, Lee EJ, Cho KS, Yoon SJ, Lee YH, Hong SJ. Preventive effects of oligomerized polyphenol on estradiol-induced prostatitis in rats. Yonsei Med J. 2009; 50:391–8.
Article9. Seo SI, Lee SJ, Kim JC, Choi YJ, SW SW, Hwang TK, et al. Effects of androgen deprivation on chronic bacterial prostatitis in a rat model. Int J Urol. 2003; 10:485–91.
Article10. Gerard-Monnier D, Erdelmeier I, Regnard K, Moze-Henry N, Yadan JC, Chaudiere J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998; 11:1176–83.11. Kang HS, Chung HY, Jung JH, Son BW, Choi JS. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem Pharm Bull (Tokyo). 2003; 51:1012–4.
Article12. Kang KA, Lee KH, Chae S, Koh YS, Yoo BS, Kim JH, et al. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic Res. 2005; 39:883–92.13. Wijesekara I, Yoon NY, Kim SK. Phlorotannins from Ecklonia cava (Phaeophyceae): biological activities and potential health benefits. Biofactors. 2010; 36:408–14.
Article14. Shull JD, Gorski J. Regulation of prolactin gene transcription in vivo: interactions between estrogen, pimozide, and alpha-er-gocryptine. Mol Pharmacol. 1990; 37:215–21.15. Tangbanluekal L, Robinette CL. Prolactin mediates estradiol-induced inflammation in the lateral prostate of Wistar rats. Endocrinology. 1993; 132:2407–16.
Article16. Wilson MJ, Woodson M, Wiehr C, Reddy A, Sinha AA. Matrix metalloproteinases in the pathogenesis of estradiol-induced nonbacterial prostatitis in the lateral prostate lobe of the Wistar rat. Exp Mol Pathol. 2004; 77:7–17.
Article17. Arakawa S, Matsui T, Gohji K, Okada H, Kamidono S. Prostatitis–the Japanese viewpoint. Int J Antimicrob Agents. 1999; 11:201–3.
Article18. Maher J, Yamamoto M. The rise of antioxidant signaling–the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010; 244:4–15.
Article19. Heo SJ, Park PJ, Park EJ, Kim SK, Jeon YJ. Antioxidant activity of enzymatic extracts from a brown seaweed Ecklonia cava by electron spin resonance spectrometry and comet assay. Eur Food Res Technol. 2005; 221:41–7.
Article20. Yasmeen T, Qureshi GS, Perveen S. Adverse effects of diclofenac sodium on renal parenchyma of adult albino rats. J Pak Med Assoc. 2007; 57:349–51.21. Van Hecken A, Schwartz JI, Depre M, De Lepeleire I, Dallob A, Tanaka W, et al. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol. 2000; 40:1109–20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Changes of T-cell Subclones in Patients with Chronic Nonbacterial Prostatitis
- Sperm Motility in Chronic Nonbacterial Prostatitis: Related to Antisperm Antibody and Seminal Cytokine and Seminal Plasma Composition
- The Anti-Inflammatory Effects of a New Herbal Formula (WSY-1075) in a Nonbacterial Prostatitis Rat Model
- Treatment of Chronic Bacterial and Nonbacterial Prostatitis by Local Injection of Amikacin Sulfate
- Urodynamic Findings of Chronic Nonbacterial Prostatitis Patients with Urinary Symptoms