Tuberc Respir Dis.
2015 Jul;78(3):210-217. 10.4046/trd.2015.78.3.210.
Effects of Lupenone, Lupeol, and Taraxerol Derived from Adenophora triphylla on the Gene Expression and Production of Airway MUC5AC Mucin
- Affiliations
-
- 1Department of Pharmacology, Chungnam National University School of Medicine, Daejeon, Korea. LCJ123@cnu.ac.kr
- 2Division of Bioscience, Dongguk University, Gyeongju, Korea.
- 3Department of Pharmacy, College of Pharmacy, Seoul National University, Seoul, Korea.
- KMID: 2320645
- DOI: http://doi.org/10.4046/trd.2015.78.3.210
Abstract
- BACKGROUND
Adenophora triphylla var. japonica is empirically used for controlling airway inflammatory diseases in folk medicine. We evaluated the gene expression and production of mucin from airway epithelial cells in response to lupenone, lupeol and taraxerol derived from Adenophora triphylla var. japonica.
METHODS
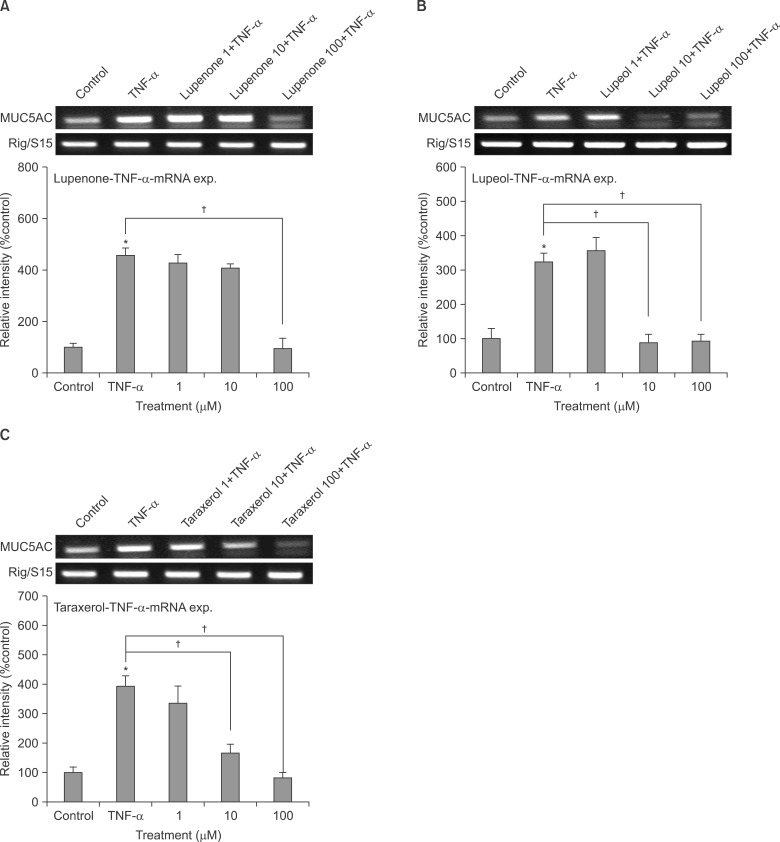
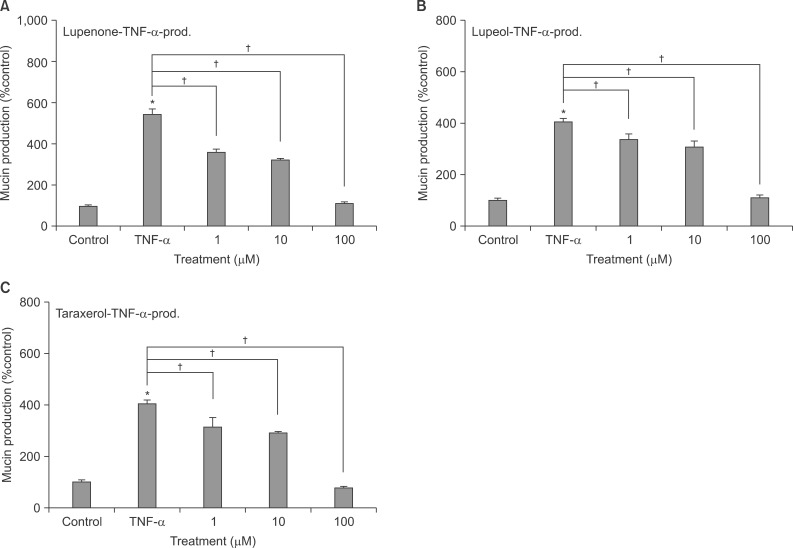
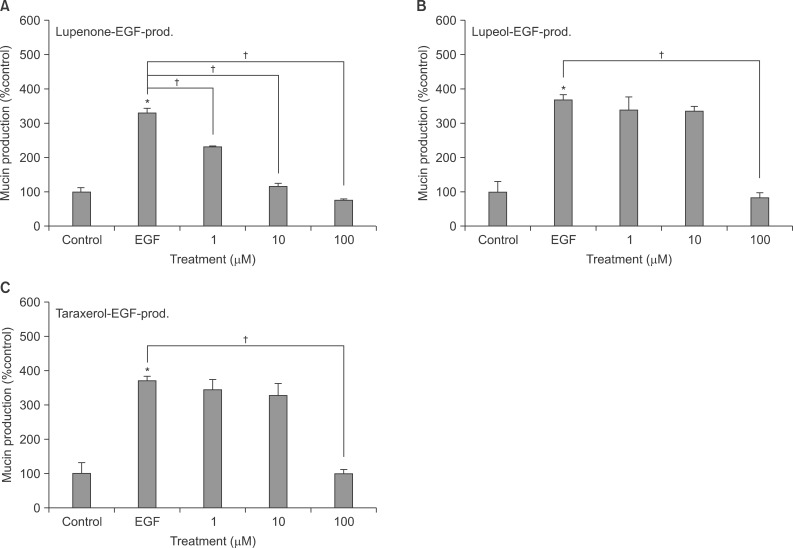
Confluent NCI-H292 cells were pretreated with lupenone, lupeol or taraxerol for 30 minutes and then stimulated with tumor necrosis factor alpha (TNF-alpha) for 24 hours. The MUC5AC mucin gene expression and production were measured by reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. Additionally, we examined whether lupenone, lupeol or taraxerol affects MUC5AC mucin production induced by epidermal growth factor (EGF) and phorbol 12-myristate 13-acetate (PMA), the other 2 stimulators of airway mucin production.
RESULTS
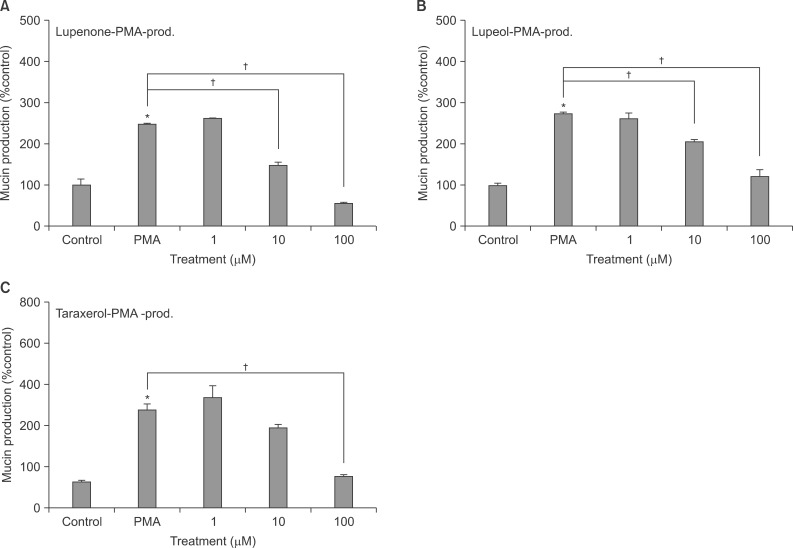
Lupenone, lupeol, and taraxerol inhibited the gene expression and production of MUC5AC mucin induced by TNF-alpha from NCI-H292 cells, respectively. The 3 compounds inhibited the EGF or PMA-induced production of MUC5AC mucin in NCI-H292 cells.
CONCLUSION
These results indicated that lupenone, lupeol and taraxerol derived from Adenophora triphylla var. japonica regulates the production and gene expression of mucin, by directly acting on airway epithelial cells. In addition, the results partly explain the mechanism of of Adenophora triphylla var. japonica as a traditional remedy for diverse inflammatory pulmonary diseases.
MeSH Terms
Figure
Reference
-
1. Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009; 135:505–512. PMID: 19201713.
Article2. Heo HJ, Kim C, Lee HJ, Kim YS, Kang SS, Seo UK, et al. Carbenoxolone and triterpenoids inhibited mucin secretion from airway epithelial cells. Phytother Res. 2007; 21:462–465. PMID: 17262888.
Article3. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009; 23:1458–1461. PMID: 19288529.
Article4. Lee HJ, Lee SY, Lee MN, Kim JH, Chang GT, Seok JH, et al. Inhibition of secretion, production and gene expression of mucin from cultured airway epithelial cells by prunetin. Phytother Res. 2011; 25:1196–1200. PMID: 21305630.
Article5. Jang IM. Treatise on Asian herbal medicines. Seoul: Haksul-pyunsu-kwan in Research Institute of Natural Products of Seoul National University;2003.6. Jin SE, Son YK, Min BS, Jung HA, Choi JS. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch Pharm Res. 2012; 35:823–837. PMID: 22644850.
Article7. Kang SC, Lim SY, Song YJ. Lupeol is one of active components in the extract of Chrysanthemum indicum Linne that inhibits LMP1-induced NF-kappaB activation. PLoS One. 2013; 8:e82688. PMID: 24303085.8. Yao X, Li G, Bai Q, Xu H, Lu C. Taraxerol inhibits LPS-induced inflammatory responses through suppression of TAK1 and Akt activation. Int Immunopharmacol. 2013; 15:316–324. PMID: 23333629.
Article9. Srivastava P, Jyotshna , Gupta N, Maurya AK, Shanker K. New anti-inflammatory triterpene from the root of Ricinus communis. Nat Prod Res. 2014; 28:306–311. PMID: 24279342.10. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006; 38:116–125. PMID: 16581697.
Article11. Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997; 94:967–972. PMID: 9023366.12. Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000; 164:1546–1552. PMID: 10640773.
Article13. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003; 100:11618–11623. PMID: 12972643.14. Sakurai N, Yaguchi Y, Inoue T. Triterpenoids from Myrica rubra. Phytochemistry. 1986; 26:217–219.15. Konno C, Saito T, Oshima Y, Hikino H, Kabuto C. Structure of methyl adenophorate and triphyllol, triterpenoids of Adenophora triphylla var. japonica roots. Planta Med. 1981; 42:268–274. PMID: 17401973.16. Ahmad VU, Bano S, Mohammad FV. Nepehinol: a new triterpene from Nepeta Hindostana. Planta Med. 1985; 51:521–523. PMID: 17345277.17. Fischer BM, Rochelle LG, Voynow JA, Akley NJ, Adler KB. Tumor necrosis factor-alpha stimulates mucin secretion and cyclic GMP production by guinea pig tracheal epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999; 20:413–422. PMID: 10030839.18. Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003; 278:23243–23250. PMID: 12690113.19. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999; 96:3081–3086. PMID: 10077640.
Article20. Cohn L, Whittaker L, Niu N, Homer RJ. Cytokine regulation of mucus production in a model of allergic asthma. Novartis Found Symp. 2002; 248:201–213. PMID: 12568496.
Article21. Hong DH, Petrovics G, Anderson WB, Forstner J, Forstner G. Induction of mucin gene expression in human colonic cell lines by PMA is dependent on PKC-epsilon. Am J Physiol. 1999; 277(5 Pt 1):G1041–G1047. PMID: 10564110.22. Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004; 344:683–695. PMID: 15533438.23. Park SJ, Kang SY, Kim NS, Kim HM. Phosphatidylinositol 3-kinase regulates PMA-induced differentiation and superoxide production in HL-60 cells. Immunopharmacol Immunotoxicol. 2002; 24:211–226. PMID: 12066848.
Article24. Kim KD, Lee HJ, Lim SP, Sikder A, Lee SY, Lee CJ. Silibinin regulates gene expression, production and secretion of mucin from cultured airway epithelial cells. Phytother Res. 2012; 26:1301–1307. PMID: 22275269.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Homogentisic Acid and Natural Products Derived from Pinellia ternata on Secretion, Production and Gene Expression of MUC5AC Mucin from Cultured Airway Epithelial Cells
- Effects of Cynaroside, Cynarin and Linarin on Secretion, Production and Gene Expression of Airway MUC5AC Mucin in NCI-H292 Cells
- Growth Factor- and Phorbol Ester-induced Production and Gene Expression of MUC5AC Mucin in Human Airway Epithelial NCI-H292 Cells Were Inhibited by Afzelin and Natural Products Derived from Houttuynia Cordata
- Effects of Caffeic Acid, Myristicin and Rosemarinic Acid on the Gene Expression and Production of Airway MUC5AC Mucin
- Effects of Nodakenin, Columbianadin, and Umbelliferone Isolated from the Roots of Angelica decursiva on the Gene Expression and Production of MUC5AC Mucin from Human Airway Epithelial NCI-H292 Cells