Nat Prod Sci.
2017 Sep;23(3):201-207. 10.20307/nps.2017.23.3.201.
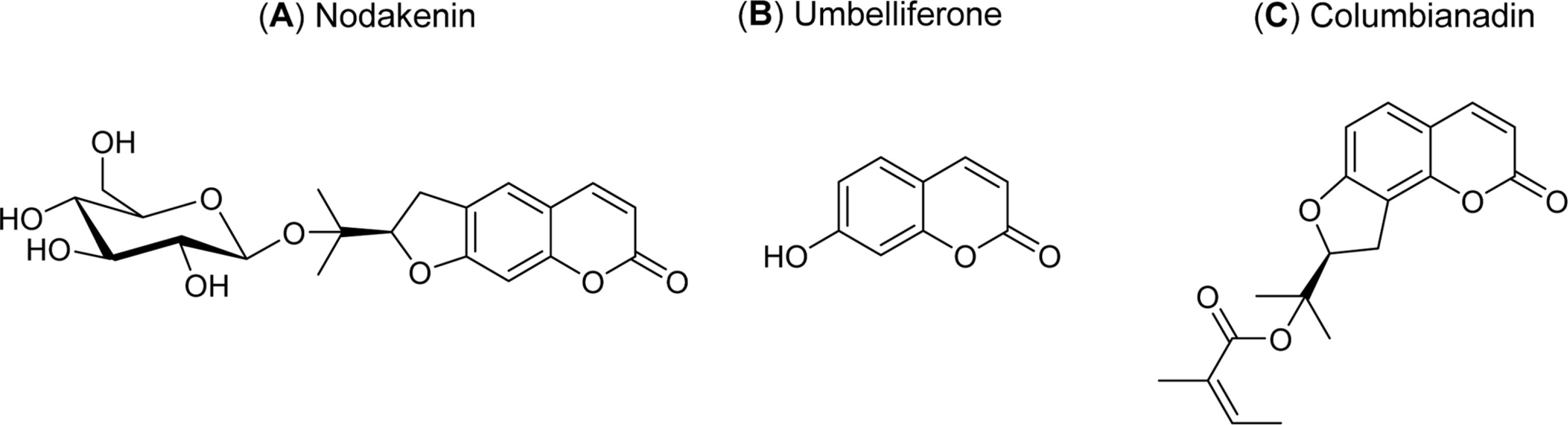
Effects of Nodakenin, Columbianadin, and Umbelliferone Isolated from the Roots of Angelica decursiva on the Gene Expression and Production of MUC5AC Mucin from Human Airway Epithelial NCI-H292 Cells
- Affiliations
-
- 1Department of Health Management and Smith Liberal Arts College, Sahmyook University, Seoul 01795, Korea.
- 2Department of Pharmacology, School of Medicine, Chungnam National University, Daejeon 35015, Korea. LCJ123@cnu.ac.kr
- KMID: 2393801
- DOI: http://doi.org/10.20307/nps.2017.23.3.201
Abstract
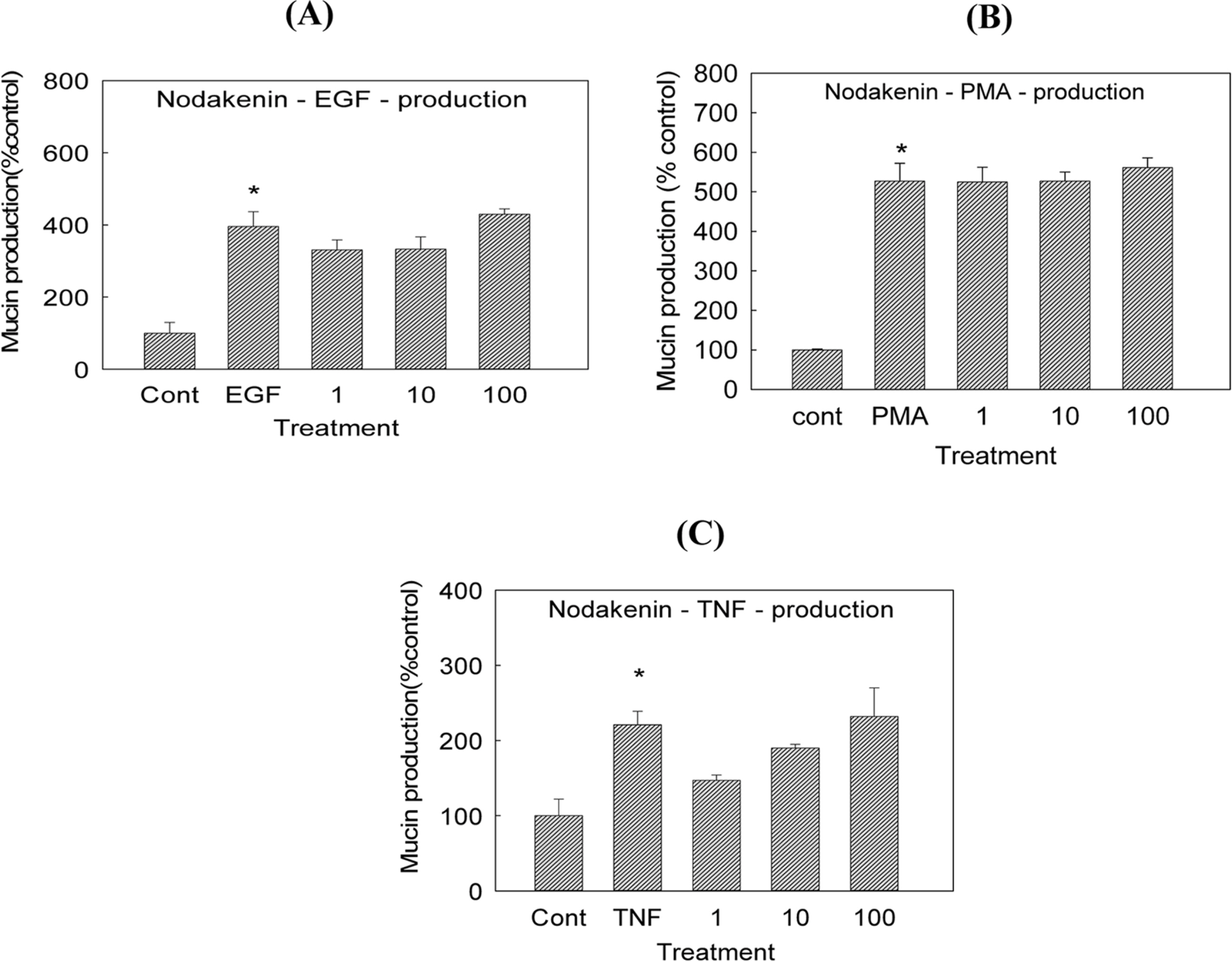
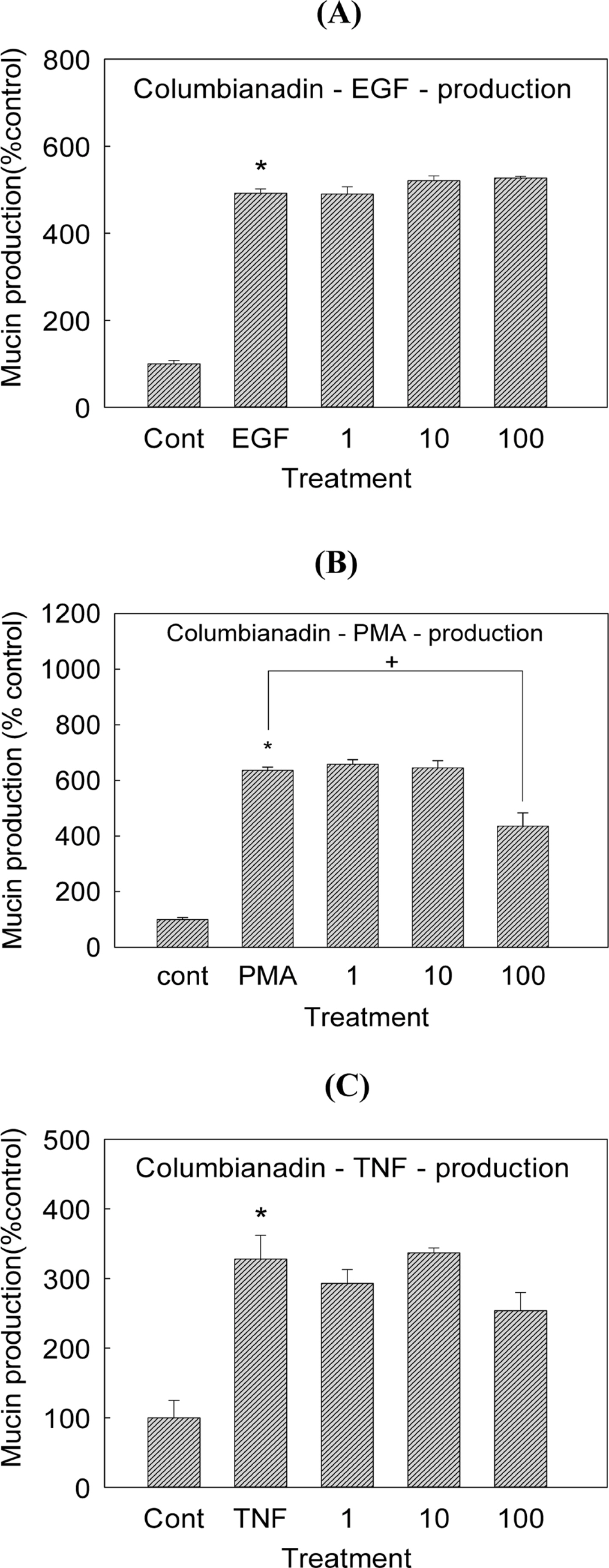
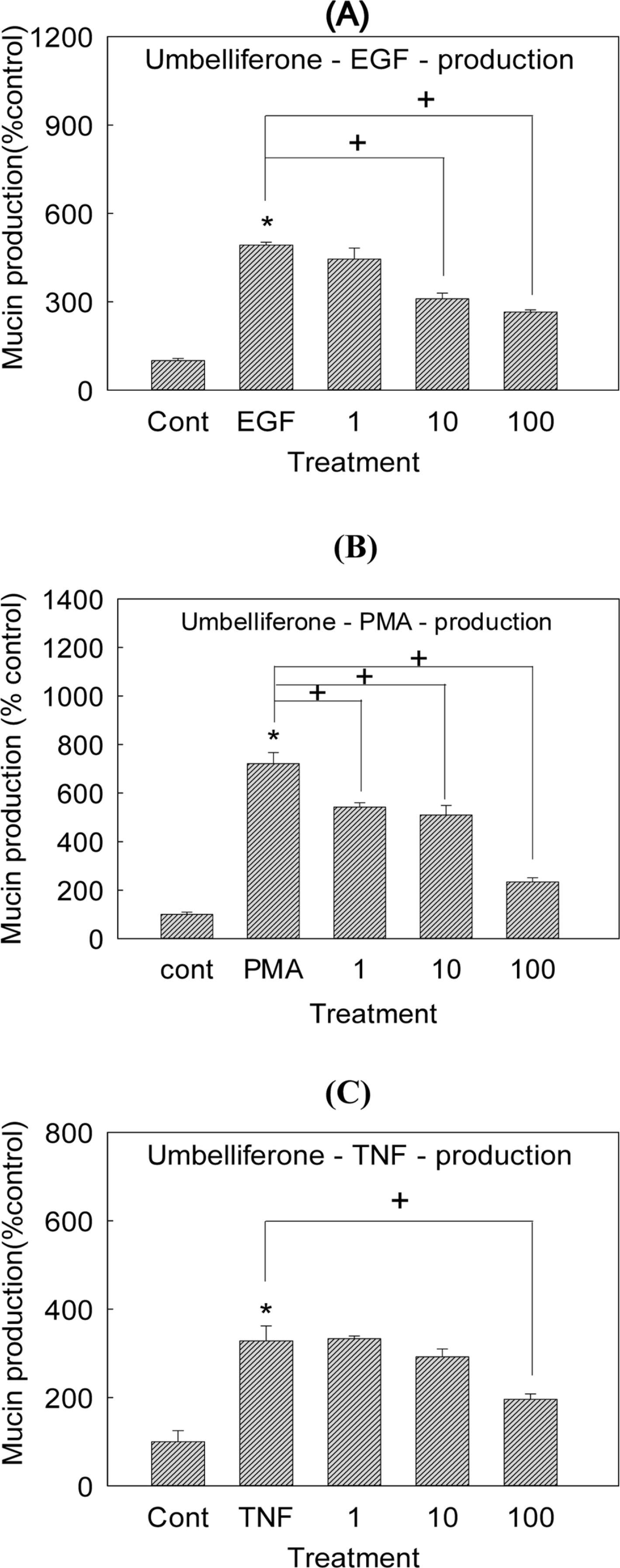
- Angelica decursiva has been utilised as remedy for controlling the airway inflammatory diseases in folk medicine. We investigated whether nodakenin, columbianadin, and umbelliferone isolated from the roots of Angelica decursiva inhibit the gene expression and production of MUC5AC mucin from human airway epithelial cells. Confluent NCI-H292 cells were pretreated with nodakenin, columbianadin or umbelliferone for 30 min and then stimulated with epidermal growth factor (EGF), phorbol 12-myristate 13-acetate (PMA) or tumor necrosis factor-α (TNF-α) for 24 h. The MUC5AC mucin gene expression was measured by reverse transcription - polymerase chain reaction (RT-PCR). Production of MUC5AC mucin protein was measured by enzyme-linked immunosorbent assay (ELISA). The results were as follows: (1) Nodakenin did not affect the expression of MUC5AC mucin gene induced by EGF, PMA or TNF-α. Columbianadin inhibited the expression of MUC5AC mucin gene induced by EGF or PMA. However, umbelliferone inhibited the expression of MUC5AC mucin gene induced by EGF, PMA or TNF-α; (2) Nodakenin also did not affect the production of MUC5AC mucin protein induced by EGF, PMA or TNF-α. Columbianadin inhibited the production of MUC5AC mucin protein induced by PMA. However, umbelliferone inhibited the production of MUC5AC mucin protein induced by EGF, PMA or TNF-α. These results suggest that, among the three compounds investigated, umbelliferone only inhibits the gene expression and production of MUC5AC mucin stimulated by various inducers, by directly acting on airway epithelial cells, and the results might explain the traditional use of Angelica decursiva as remedy for diverse inflammatory pulmonary diseases.
Keyword
MeSH Terms
Figure
Reference
-
References
(1). Voynow J. A.., Rubin B. K.Chest. 2009. 135:505–512.(2). Lee H. J.., Ryu J.., Park S. H.., Seo E. K.., Han A. R.., Lee S. K.., Kim Y. S.., Hong J. H.., Seok J. H.., Lee C. J.Arch. Pharm. Res. 2015. 38:620–627.(3). Seo H. S.., Sikder M. A.., Lee H.J.., Ryu J.., Lee C. J.Biomol. Ther (Seoul). 2014. 22:525–531.(4). Sikder M. A.., Lee H. J.., Lee S. Y.., Bae H. S.., Kim J. H.., Chang G. T.., Lee C. J.Biomol. Ther (Seoul). 2011. 19:320–323.(5). Lee H. J.., Lee S. Y.., Kim Y. S.., Jeon B. K.., Lee J. W.., Bae H. S.., Lee C. J.Biomol. Ther (Seoul). 2010. 18:396–401.(6). Lee H. J.., Lee S. Y.., Jeon B. K.., Lee J. W.., Kim Y. S.., Lee M. N.., Lee C. J.Biomol. Ther (Seoul). 2010. 18:294–299.(7). Jang I. M.Treatise on asian herbal medicines; Haksul-pyunsu-kwan in Research institute of natural products of Seoul National University: Seoul,. 2003. , p. 2759.(8). Lim H. J.., Lee J. H.., Choi J. S.., Lee S. K.., Kim Y. S.., Kim H. P. J.Ethnopharmacol. 2014. 155:1353–1361.(9). Park S. J.., Cha H. S.., Lee Y. H.., Kim W. J.., Kim D. H.., Kim E. C.., Lee K. H.., Kim T. J.Biosci. Biotechnol. Biochem. 2014. 78:1568–1571.(10). Vasconcelos J. F.., Teixeira M. M.., Barbosa-Filho J. M.., Agra M. F.., Nunes X. P.., Giulietti A. M.., Ribeiro-Dos-Santos R.., Soares M. B.Eur. J. Pharmacol. 2009. 609:126–131.(11). Xiong Y.., Wang J.., Yu H.., Zhang X.., Miao C.., Ma S.Immunopharmacol. Immunotoxicol. 2014. 36:341–348.(12). Rogers D. F.., Barnes P. J.Ann. Med. 2006. 38:116–125.(13). Li J. D.., Dohrman A. F.., Gallup M.., Miyata S.., Gum J. R.., Kim Y. S.., Nadel J. A.., Prince A.., Basbaum C. B.Proc. Natl. Acad. Sci. USA. 1997. 94:967–972.(14). Takeyama K.., Dabbagh K.., Lee H. M.., Agusti C.., Lausier J. A.., Ueki I. F.., Grattan K. M.., Nadel J. A.Proc. Natl. Acad. Sci. USA. 1999. 96:3081–3086.(15). Shao M. X.., Ueki I. F.., Nadel J. A.Proc. Natl. Acad. Sci. USA. 2003. 100:11618–11623.(16). Song K. S.., Lee W. J.., Chung K. C.., Koo J. S.., Yang E. J.., Choi J. Y.., Yoon J. H. J.Biol. Chem. 2003. 278:23243–23250.(17). Takeyama K.., Dabbagh K.., Shim J. J.., Dao-Pick T.., Ueki I. F.., Nadel J. A. J.Immunol. 2000. 164:1546–1552.(18). Hong D. H.., Petrovics G.., Anderson W. B.., Forstner J.., Forstner G.Am. J. Physiol. 1999. 277:G1041–G1047.(19). Hewson C. A.., Edbrooke M. R.., Johnston S. L. J.Mol. Biol. 2004. 344:683–695.(20). Park S. J.., Kang S. Y.., Kim N. S.., Kim H. M.Immunopharmacol. Immunotoxicol. 2002. 24:211–226.(21). Fischer B. M.., Rochelle L. G.., Voynow J. A.., Akley N. J.., Adler K. B.Am. J. Respir. Cell Mol. Biol. 1999. 20:413–422.(22). Cohn L.., Whittaker L.., Niu N.., Homer R. J.Novartis Found. Symp. 2002. 248:201–213.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Cynaroside, Cynarin and Linarin on Secretion, Production and Gene Expression of Airway MUC5AC Mucin in NCI-H292 Cells
- Growth Factor- and Phorbol Ester-induced Production and Gene Expression of MUC5AC Mucin in Human Airway Epithelial NCI-H292 Cells Were Inhibited by Afzelin and Natural Products Derived from Houttuynia Cordata
- Effects of Homogentisic Acid and Natural Products Derived from Pinellia ternata on Secretion, Production and Gene Expression of MUC5AC Mucin from Cultured Airway Epithelial Cells
- Diclofenac Inhibits Phorbol Ester-Induced Gene Expression and Production of MUC5AC Mucin via Affecting Degradation of IkBα and Translocation of NF-kB p65 in NCI-H292 Cells
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells