Tuberc Respir Dis.
2015 Apr;78(2):99-105. 10.4046/trd.2015.78.2.99.
Regulation of CYP1A1 and Inflammatory Cytokine by NCOA7 Isoform 4 in Response to Dioxin Induced Airway Inflammation
- Affiliations
-
- 1Regional Center for Respiratory Diseases, Kangwon National University Hospital, Chuncheon, Korea. wjkim47@gmail.com
- 2Department of Internal Medicine and Environmental Health Center, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea.
- KMID: 2320601
- DOI: http://doi.org/10.4046/trd.2015.78.2.99
Abstract
- BACKGROUND
Aryl hydrocarbon receptor (AhR), a ligand-dependent transcription factor, binds to a wide variety of synthetic and naturally occurring compounds. AhR is involved in the regulation of inflammatory response during acute and chronic respiratory diseases. We investigated whether nuclear receptor coactivator 7 (NCOA7) could regulate transcriptional levels of AhR target genes and inflammatory cytokines in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated human bronchial epithelial cells. This study was based on our previous study that NCOA7 was differentially expressed between normal and chronic obstructive pulmonary disease lung tissues.
METHODS
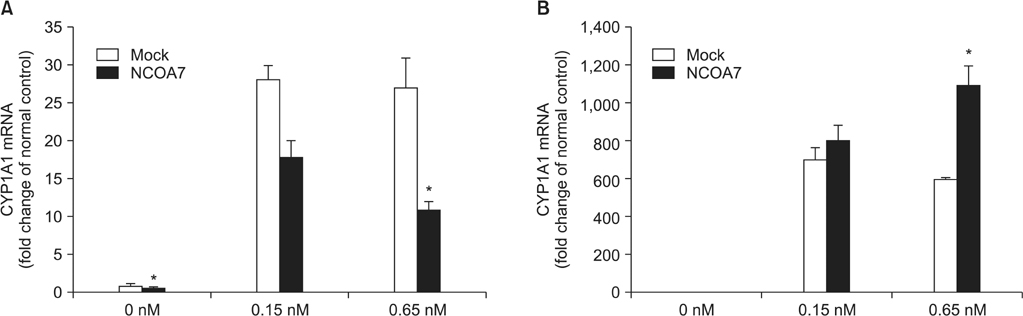
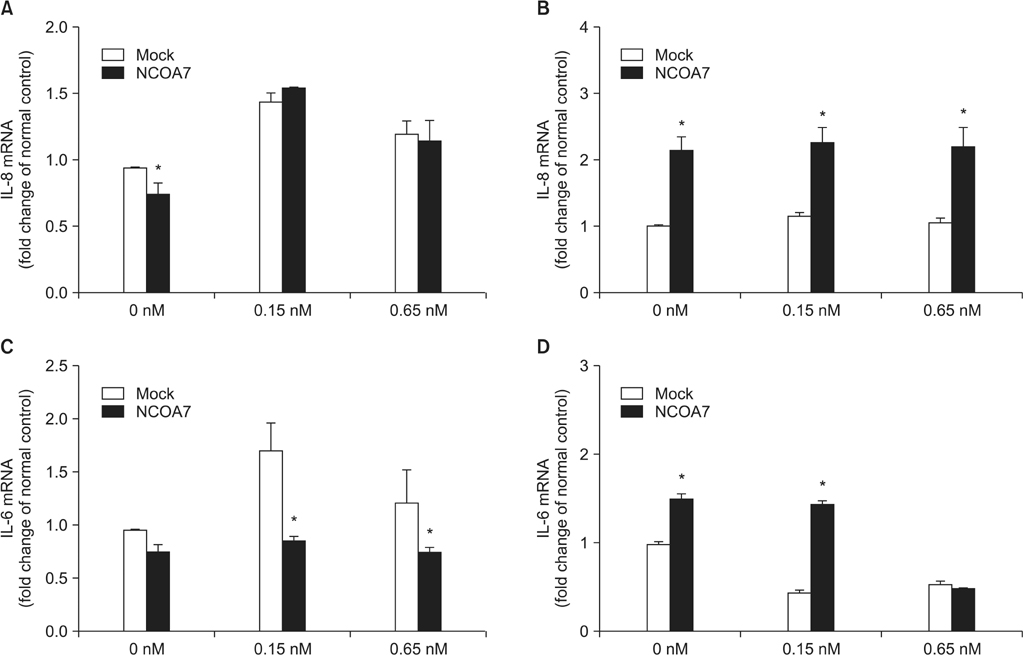
BEAS-2B and A549 cells grown under serum-free conditions were treated with or without TCDD (0.15 nM and 6.5 nM) for 24 hours after transfection of pCMV-NCOA7 isoform 4. Expression levels of cytochrome P4501A1 (CYP1A1), IL-6, and IL-8 were measured by quantitative real-time polymerase chain reaction.
RESULTS
The transcriptional activities of CYP1A1 and inflammatory cytokines were strongly induced by TCDD treatment in both BEAS-2B and A549 cell lines. The NCOA7 isoform 4 oppositely regulated the transcriptional activities of CYP1A1 and inflammatory cytokines between BEAS-2B and A549 cell lines.
CONCLUSION
Our results suggest that NCOA7 could act as a regulator in the TCDD-AhR signaling pathway with dual roles in normal and abnormal physiological conditions.
Keyword
MeSH Terms
-
Cell Line
Cytochrome P-450 CYP1A1*
Cytochromes
Cytokines
Dioxins
Epithelial Cells
Humans
Inflammation*
Interleukin-6
Interleukin-8
Lung
Pulmonary Disease, Chronic Obstructive
Real-Time Polymerase Chain Reaction
Receptors, Aryl Hydrocarbon
Tetrachlorodibenzodioxin
Transcription Factors
Transfection
Cytochrome P-450 CYP1A1
Cytochromes
Cytokines
Dioxins
Interleukin-6
Interleukin-8
Receptors, Aryl Hydrocarbon
Tetrachlorodibenzodioxin
Transcription Factors
Figure
Reference
-
1. Das SK. Harmful health effects of cigarette smoking. Mol Cell Biochem. 2003; 253:159–165.2. Kitamura M, Kasai A. Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett. 2007; 252:184–194.3. Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982; 22:517–554.4. Lai H, Rogers DF. New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: targeting intracellular signaling pathways. J Aerosol Med Pulm Drug Deliv. 2010; 23:219–231.5. Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008; 8:183–192.6. Mauad T, Dolhnikoff M. Pathologic similarities and differences between asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008; 14:31–38.7. Hylkema MN, Sterk PJ, de Boer WI, Postma DS. Tobacco use in relation to COPD and asthma. Eur Respir J. 2007; 29:438–445.8. Lai ZW, Hundeiker C, Gleichmann E, Esser C. Cytokine gene expression during ontogeny in murine thymus on activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1997; 52:30–37.9. Vogel C, Abel J. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on growth factor expression in the human breast cancer cell line MCF-7. Arch Toxicol. 1995; 69:259–265.10. Vogel C, Donat S, Dohr O, Kremer J, Esser C, Roller M, et al. Effect of subchronic 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on immune system and target gene responses in mice: calculation of benchmark doses for CYP1A1 and CYP1A2 related enzyme activities. Arch Toxicol. 1997; 71:372–382.11. Warren TK, Mitchell KA, Lawrence BP. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung. Toxicol Sci. 2000; 56:114–123.12. Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003; 1619:263–268.13. Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003; 43:309–334.14. Tojo M, Matsuzaki K, Minami T, Honda Y, Yasuda H, Chiba T, et al. The aryl hydrocarbon receptor nuclear transporter is modulated by the SUMO-1 conjugation system. J Biol Chem. 2002; 277:46576–46585.15. Beischlag TV, Wang S, Rose DW, Torchia J, Reisz-Porszasz S, Muhammad K, et al. Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex. Mol Cell Biol. 2002; 22:4319–4333.16. Kasai A, Hiramatsu N, Hayakawa K, Yao J, Maeda S, Kitamura M. High levels of dioxin-like potential in cigarette smoke evidenced by in vitro and in vivo biosensing. Cancer Res. 2006; 66:7143–7150.17. Gebremichael A, Tullis K, Denison MS, Cheek JM, Pinkerton KE. Ah-receptor-dependent modulation of gene expression by aged and diluted sidestream cigarette smoke. Toxicol Appl Pharmacol. 1996; 141:76–83.18. Shao W, Halachmi S, Brown M. ERAP140, a conserved tissue-specific nuclear receptor coactivator. Mol Cell Biol. 2002; 22:3358–3372.19. Paramanik V, Thakur MK. Interaction of estrogen receptor associated protein (ERAP) 140 with ER beta decreases but its expression increases in aging mouse cerebral cortex. Cell Mol Neurobiol. 2010; 30:961–966.20. Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci U S A. 1997; 94:8479–8484.21. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995; 83:835–839.22. Naar AM, Beaurang PA, Zhou S, Abraham S, Solomon W, Tjian R. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature. 1999; 398:828–832.23. Norman AW, Litwack G. Estrogens and progestins. In : Litwack G, editor. Hormones. London: Academic Press;1987. p. 550–560.24. Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009; 77:734–745.25. Badal S, Delgoda R. Role of the modulation of CYP1A1 expression and activity in chemoprevention. J Appl Toxicol. 2014; 34:743–753.26. Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003; 144:3382–3398.27. Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005; 33:489–494.28. Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer. 2009; 9:187.29. Buterin T, Hess MT, Luneva N, Geacintov NE, Amin S, Kroth H, et al. Unrepaired fjord region polycyclic aromatic hydrocarbon-DNA adducts in ras codon 61 mutational hot spots. Cancer Res. 2000; 60:1849–1856.30. Rodriguez M, Potter DA. CYP1A1 regulates breast cancer proliferation and survival. Mol Cancer Res. 2013; 11:780–792.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhancement of Allergen-induced Airway Inflammation by NOX2 Deficiency
- Epithelial PI3K-δ Promotes House Dust Mite-Induced Allergic Asthma in NLRP3 Inflammasome-Dependent and -Independent Manners
- Novel Drugs for Asthma Treatment: Immunomodulatory Therapy
- Autophagy Enhancers Regulate Cholesterol-Induced Cytokine Secretion and Cytotoxicity in Macrophages
- 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Regulates the Expression of Aryl Hydrocarbon Receptor-Related Factors and Cytokines in Peripheral Blood Mononuclear Cells and CD4+ T cells from Patients with Atopic Dermatitis and Psoriasis