Tuberc Respir Dis.
2013 Nov;75(5):205-209.
Effect of Prunetin on TNF-alpha-Induced MUC5AC Mucin Gene Expression, Production, Degradation of IkappaB and Translocation of NF-kappaB p65 in Human Airway Epithelial Cells
- Affiliations
-
- 1Department of Pharmacology, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea. LCJ123@cnu.ac.kr
- 2Pulmonology Section, Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
Abstract
- BACKGROUND
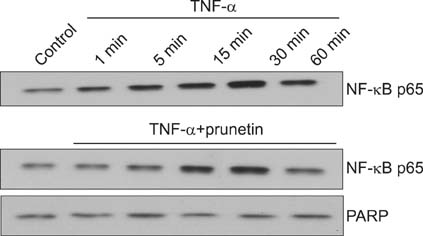
We investigated whether prunetin significantly affects tumor necrosis factor-alpha (TNF-alpha)-induced MUC5AC mucin gene expression, production, inhibitory kappa B (IkappaB) degradation and nuclear factor kappa B (NF-kappaB) p65 translocation in human airway epithelial cells.
METHODS
Confluent NCI-H292 cells were pretreated with prunetin for 30 minutes and then stimulated with TNF-alpha for 24 hours or the indicated periods. MUC5AC mucin gene expression and mucin protein production were measured by reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. The effect of prunetin on TNF-alpha-induced degradation of IkappaB and translocation of NF-kappaB p65 was investigated by western blot analysis.
RESULTS
We found that incubation of NCI-H292 cells with prunetin significantly inhibited mucin production and down-regulated the MUC5AC gene expression induced by TNF-alpha. Prunetin inhibited TNF-alpha-induced degradation of IkappaB and translocation of NF-kappaB p65.
CONCLUSION
This result suggests that prunetin inhibits the NF-kappaB signaling pathway, which may explain its role in the inhibition of MUC5AC mucin gene expression and production regulated by the NF-kappaB signaling pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Adler KB, Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. Am J Respir Cell Mol Biol. 2001; 25:397–400.2. Basbaum C, Lemjabbar H, Longphre M, Li D, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med. 1999; 160(5 Pt 2):S44–S48.3. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009; 23:1458–1461.4. Lee HJ, Lee SY, Bae HS, Kim JH, Chang GT, Seok JH, et al. Inhibition of airway MUC5AC mucin production and gene expression induced by epidermal growth factor or phorbol ester by glycyrrhizin and carbenoxolone. Phytomedicine. 2011; 18:743–747.5. Lee HJ, Lee SY, Jeon BK, Lee JW, Kim YS, Lee MN, et al. Effect of platycodin D on airway MUC5AC mucin production and gene expression induced by growth factor and proinflammatory factor. Biomol Ther. 2010; 18:294–299.6. Lee HJ, Lee SY, Lee MN, Kim JH, Chang GT, Seok JH, et al. Inhibition of secretion, production and gene expression of mucin from cultured airway epithelial cells by prunetin. Phytother Res. 2011; 25:1196–1200.7. Jung HA, Kim AR, Chung HY, Choi JS. In vitro antioxidant activity of some selected Prunus species in Korea. Arch Pharm Res. 2002; 25:865–872.8. Keung WM. Biochemical studies of a new class of alcohol dehydrogenase inhibitors from Radix puerariae. Alcohol Clin Exp Res. 1993; 17:1254–1260.9. Ko WC, Shih CM, Lai YH, Chen JH, Huang HL. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem Pharmacol. 2004; 68:2087–2094.10. Sheikh S, Weiner H. Allosteric inhibition of human liver aldehyde dehydrogenase by the isoflavone prunetin. Biochem Pharmacol. 1997; 53:471–478.11. Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997; 94:967–972.12. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999; 96:3081–3086.13. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003; 100:11618–11623.14. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006; 38:116–125.15. Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003; 278:23243–23250.16. Fischer BM, Rochelle LG, Voynow JA, Akley NJ, Adler KB. Tumor necrosis factor-alpha stimulates mucin secretion and cyclic GMP production by guinea pig tracheal epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999; 20:413–422.17. Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002; 2:725–734.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apigenin Inhibits Tumor Necrosis Factor-alpha-Induced Production and Gene Expression of Mucin through Regulating Nuclear Factor-Kappa B Signaling Pathway in Airway Epithelial Cells
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Diclofenac Inhibits Phorbol Ester-Induced Gene Expression and Production of MUC5AC Mucin via Affecting Degradation of IkBα and Translocation of NF-kB p65 in NCI-H292 Cells
- Regulation of Tumor Necrosis Factor-alpha-induced Airway Mucin Production and Gene Expression by Carbenoxolone, Prunetin, and Silibinin
- Involvement of IKK/IkBα/NF-kB p65 Signaling into the Regulative Effect of Engeletin on MUC5AC Mucin Gene Expression in Human Airway Epithelial Cells