Tuberc Respir Dis.
2009 Dec;67(6):569-573.
A Case of Idiopathic Pulmonary Alveolar Proteinosis Treated with Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) after Partial Response to Whole Lung Lavage
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Masan Samsung Hospital, Sungkyunkwan University School of Medicine, Masan, Korea. kangkw9@naver.com
Abstract
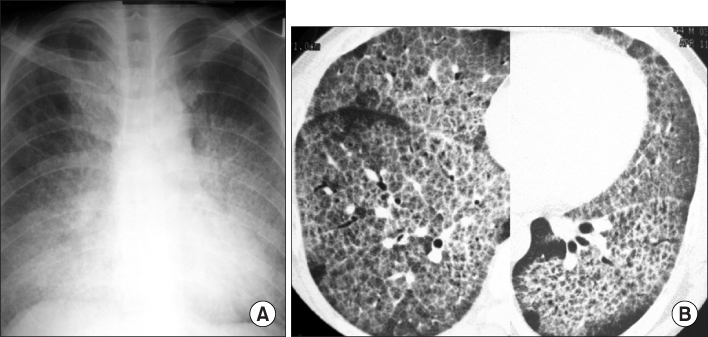
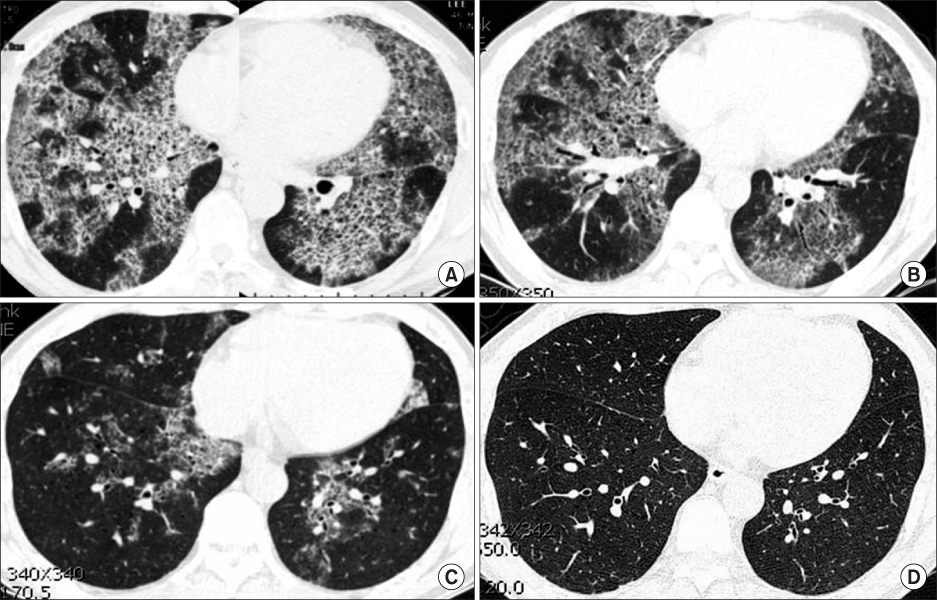
- Idiopathic pulmonary alveolar proteinosis (PAP) is a rare disorder characterized by surfactant component accumulation in the alveolar space. Idiopathic PAP has recently been recognized as a autoimmune disease of impaired alveolar macrophage function caused by autoantibodies against granulocyte-macrophage colony-stimulating factor (GM-CSF). While whole lung lavage has been the standard treatment, not every patient shows a complete response. Subcutaneous injection or inhalation of GM-CSF is another promising treatment option for PAP. A 45-year-old patient visited our hospital for dyspnea, he was diagnosed as PAP and underwent whole lung lavage. Eighteen months later, the patient had not achieved complete remission in despite of initial response. After then he was administered with GM-CSF (5 microgram/kg/day, subcutaneous injection) for fivetimes a week during 2 months. Nine months later, the abnormal shadows in high-resolution computed tomography (HRCT) decreased and the patient fully recovered in forced vital capacity. After 60 months, the HRCT scan showed complete remission of PAP.
Keyword
MeSH Terms
-
Autoantibodies
Autoimmune Diseases
Bronchoalveolar Lavage
Colony-Stimulating Factors
Dyspnea
Granulocyte-Macrophage Colony-Stimulating Factor
Humans
Inhalation
Injections, Subcutaneous
Lung
Macrophages, Alveolar
Middle Aged
Pulmonary Alveolar Proteinosis
Vital Capacity
Autoantibodies
Colony-Stimulating Factors
Granulocyte-Macrophage Colony-Stimulating Factor
Figure
Reference
-
1. Golde DW, Territo M, Finley TN, Cline MJ. Defective lung macrophages in pulmonary alveolar proteinosis. Ann Intern Med. 1976. 85:304–309.2. Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994. 91:5592–5596.3. Kavuru MS, Sullivan EJ, Piccin R, Thomassen MJ, Stoller JK. Exogenous granulocyte-macrophage colony stimulating factor administration for pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000. 161:1143–1148.4. Seymour JF, Presneill JJ, Schoch OD, Downie GH, Moore PE, Doyle IR, et al. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am J Respir Crit Care Med. 2001. 163:524–531.5. Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, Czich C, et al. An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest. 2006. 130:227–237.6. Robb L, Drinkwater CC, Metcalf D, Li R, Kontgen F, Nicola NA. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colonystimulating factor and interleukins 3 and 5. Proc Natl Acad Sci U S A. 1995. 92:9565–9569.7. Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003. 349:2527–2539.8. Kitamura T, Tanaka N, Watanabe J, Uchida , Kanegasaki S, Yamada Y, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999. 190:875–880.9. Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MS, et al. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2003. 285:L1132–L1136.10. Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001. 15:557–567.11. Kim G, Lee SJ, Lee HP, Yoo CG, Han SK, Shim YS, et al. The clinical characteristics of pulmonary alveolar proteinosis: experience at Seoul National University Hospital, and review of the literature. J Korean Med Sci. 1999. 14:159–164.12. Nam SB, Park KY, Lee HJ, Jung JW, Choi YH, Kim HS, et al. A case of pulmonary alveolar proteinosis with spontaneous resolution. Tuberc Respir Dis. 2007. 63:294–298.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Pulmonary Alveolar Proteinosis that Improved with GM-CSF Inhalation Therapy
- Whole lung lavage with granulocyte macrophage colony-stimulating factor inhalation in children with pulmonary alveolar proteinosis: A case report
- A Case of Pulmonary Alveolar Proteinosis Improved with Inhaled Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
- A Case of Pulmonary Alveolar Proteinosis with Spontaneous Resolution
- Plasma G-CSF and GM-CSF Concentrations and Expression of their Receptors on the Granulocyte in Children with Leukocytosis