Korean J Urol.
2013 Nov;54(11):721-731.
Androgens Modulate Endothelial Function and Endothelial Progenitor Cells in Erectile Physiology
- Affiliations
-
- 1Department of Biochemistry, Boston University School of Medicine, Boston, MA, USA. atraish@bu.edu
- 2Department of Urology, Boston University School of Medicine, Boston, MA, USA.
- 3Division of Graduate Medical Sciences, Boston University School of Medicine, Boston, MA, USA.
Abstract
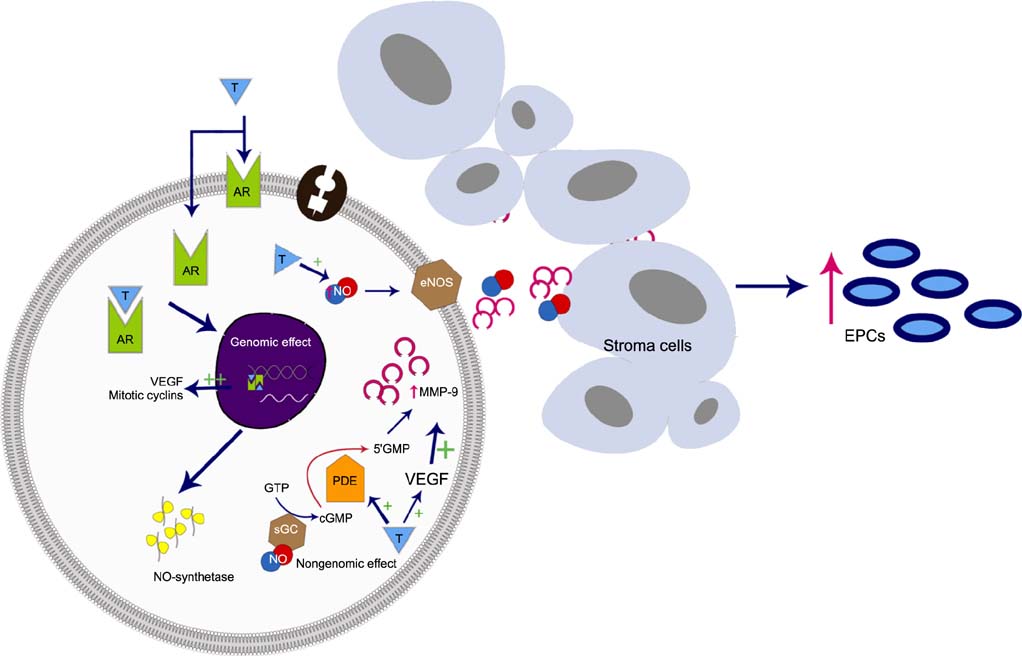
- The incidence of erectile dysfunction (ED) increases with age and cardiovascular disease risk factors, such as hypertension, hyperlipidemia, insulin resistance, obesity, and diabetes. These risk factors are thought to contribute to endothelial dysfunction and atherosclerosis, thus contributing to the pathophysiology of ED. The role of the endothelium in regulating erectile physiology is well established. However, the role of androgens in modulating endothelial function and endothelial repair mechanisms subsequent to vascular injury in erectile tissue remains a subject of intensive research. The clinical and preclinical evidence discussed in this review suggests that androgens regulate endothelial function and also play an important role in the development and maturation of endothelial progenitor cells (EPCs), which are thought to play a critical role in repair of endothelial injury in vascular beds. In this review, we discuss the data available on the effects of androgens on endothelial function and EPCs in the repair of vascular injury. Indeed, more research is needed to fully understand the molecular and cellular basis of androgen action in regulating the development, differentiation, maturation, migration, and homing of EPCs to the site of injury. A better understanding of these processes will be critical to the development of new therapeutic approaches to the treatment of vascular ED.
MeSH Terms
Figure
Reference
-
1. Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003; 170(2 Pt 2):S6–S13.2. Azadzoi KM, Kim N, Brown ML, Goldstein I, Cohen RA, Saenz de Tejada I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J Urol. 1992; 147:220–225.3. Kim N, Azadzoi KM, Goldstein I, Saenz de Tejada I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J Clin Invest. 1991; 88:112–118.4. Saenz de Tejada I, Goldstein I, Krane RJ. Local control of penile erection. Nerves, smooth muscle, and endothelium. Urol Clin North Am. 1988; 15:9–15.5. Saenz de Tejada I, Kim N, Lagan I, Krane RJ, Goldstein I. Regulation of adrenergic activity in penile corpus cavernosum. J Urol. 1989; 142:1117–1121.6. Saenz de Tejada I, Angulo J, Cellek S, Gonzalez-Cadavid N, Heaton J, Pickard R, et al. Pathophysiology of erectile dysfunction. J Sex Med. 2005; 2:26–39.7. Castela A, Vendeira P, Costa C. Testosterone, endothelial health, and erectile function. ISRN Endocrinol. 2011; 2011:839149.8. Rosenzweig A. Endothelial progenitor cells. N Engl J Med. 2003; 348:581–582.9. Traish AM, Abu-Zahra H, Guay AT. The brain, the penis and steroid hormones: clinical correlates with endothelial dysfunction. Curr Pharm Des. 2008; 14:3723–3736.10. Fazio L, Brock G. Erectile dysfunction: management update. CMAJ. 2004; 170:1429–1437.11. Vercellotti GM, Moldow CF, Jacob HS. Complement, oxidants, and endothelial injury: how a bedside observation opened a door to vascular biology. J Clin Invest. 2012; 122:3044–3045.12. Foresta C, Di Mambro A, Caretta N, De Toni L, Zuccarello D, Ferlin A. Effect of vardenafil on endothelial progenitor cells in hypogonadotrophic hypogonadal patients: role of testosterone treatment. Clin Endocrinol (Oxf). 2009; 71:412–416.13. Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003; 23:1185–1189.14. Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003; 24:6 Suppl. S17–S37.15. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348:593–600.16. Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995; 75:191–236.17. Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol. 2007; 52:54–70.18. Bivalacqua TJ, Usta MF, Champion HC, Leungwattanakij S, Dabisch PA, McNamara DB, et al. Effect of combination endothelial nitric oxide synthase gene therapy and sildenafil on erectile function in diabetic rats. Int J Impot Res. 2004; 16:21–29.19. Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005; 32:379–395.20. Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009; 30:10–22.21. Baumhakel M, Werner N, Bohm M, Nickenig G. Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease. Eur Heart J. 2006; 27:2184–2188.22. Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, Spera G. Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol. 2010; 17:38–47.23. Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007; 30:1029–1034.24. Guay AT. ED2: erectile dysfunction=endothelial dysfunction. Endocrinol Metab Clin North Am. 2007; 36:453–463.25. Hamed S, Brenner B, Roguin A. Nitric oxide: a key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type-2. Cardiovasc Res. 2011; 91:9–15.26. Tomada N, Tomada I, Botelho F, Pacheco-Figueiredo L, Lopes T, Negrao R, et al. Endothelial function in patients with metabolic syndrome and erectile dysfunction: a question of angiopoietin imbalance? Andrology. 2013; 1:541–548.27. Pelliccione F, D'Angeli A, Filipponi S, Falone S, Necozione S, Barbonetti A, et al. Serum from patients with erectile dysfunction inhibits circulating angiogenic cells from healthy men: relationship with cardiovascular risk, endothelial damage and circulating angiogenic modulators. Int J Androl. 2012; 35:645–652.28. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999; 85:221–228.29. Basile DP, Yoder MC. Circulating and tissue resident endothelial progenitor cells. J Cell Physiol. 2014; 229:10–16.30. Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl). 2004; 82:671–677.31. Garmy-Susini B, Varner JA. Circulating endothelial progenitor cells. Br J Cancer. 2005; 93:855–858.32. Garolla A, D'Inca R, Checchin D, Biagioli A, De Toni L, Nicoletti V, et al. Reduced endothelial progenitor cell number and function in inflammatory bowel disease: a possible link to the pathogenesis. Am J Gastroenterol. 2009; 104:2500–2507.33. Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001; 103:2885–2890.34. Koutroumpi M, Dimopoulos S, Psarra K, Kyprianou T, Nanas S. Circulating endothelial and progenitor cells: Evidence from acute and long-term exercise effects. World J Cardiol. 2012; 4:312–326.35. Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens. 2012; 6:85–99.36. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004; 24:288–293.37. La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero A. Original immunophenotype of blood endothelial progenitor cells and microparticles in patients with isolated arterial erectile dysfunction and late onset hypogonadism: effects of androgen replacement therapy. Aging Male. 2011; 14:183–189.38. Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998; 92:362–367.39. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000; 95:952–958.40. Gou X, He WY, Xiao MZ, Qiu M, Wang M, Deng YZ, et al. Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl. 2011; 13:332–338.41. Esposito K, Ciotola M, Maiorino MI, Giugliano F, Autorino R, De Sio M, et al. Circulating CD34+ KDR+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med. 2009; 6:107–114.42. Schächinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004; 44:1690–1699.43. Barkin J. Erectile dysfunction and hypogonadism (low testosterone). Can J Urol. 2011; 18:Suppl. 2–7.44. Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Ferlin A, et al. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab. 2006; 91:4599–4602.45. Herrmann JL, Abarbanell AM, Weil BR, Manukyan MC, Poynter JA, Wang Y, et al. Gender dimorphisms in progenitor and stem cell function in cardiovascular disease. J Cardiovasc Transl Res. 2010; 3:103–113.46. Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002; 16:2181–2187.47. Schultheiss D, Badalyan R, Pilatz A, Gabouev AI, Schlote N, Wefer J, et al. Androgen and estrogen receptors in the human corpus cavernosum penis: immunohistochemical and cell culture results. World J Urol. 2003; 21:320–324.48. Saad F, Gooren L, Haider A, Yassin A. Effects of testosterone gel followed by parenteral testosterone undecanoate on sexual dysfunction and on features of the metabolic syndrome. Andrologia. 2008; 40:44–48.49. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013; 217:R47–R71.50. Lu YL, Kuang L, Zhu H, Wu H, Wang XF, Pang YP, et al. Changes in aortic endothelium ultrastructure in male rats following castration, replacement with testosterone and administration of 5alpha-reductase inhibitor. Asian J Androl. 2007; 9:843–847.51. Zitzmann M, Brune M, Kornmann B, Gromoll J, von Eckardstein S, von Eckardstein A, et al. The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasoreactivity. J Clin Endocrinol Metab. 2001; 86:4867–4873.52. Zitzmann M. Testosterone deficiency and treatment in older men: definition, treatment, pitfalls. Asian J Androl. 2010; 12:623–625.53. Shabsigh R, Rajfer J, Aversa A, Traish AM, Yassin A, Kalinchenko SY, et al. The evolving role of testosterone in the treatment of erectile dysfunction. Int J Clin Pract. 2006; 60:1087–1092.54. Baba K, Yajima M, Carrier S, Morgan DM, Nunes L, Lue TF, et al. Delayed testosterone replacement restores nitric oxide synthase-containing nerve fibres and the erectile response in rat penis. BJU Int. 2000; 85:953–958.55. Chamness SL, Ricker DD, Crone JK, Dembeck CL, Maguire MP, Burnett AL, et al. The effect of androgen on nitric oxide synthase in the male reproductive tract of the rat. Fertil Steril. 1995; 63:1101–1107.56. Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol. 2011; 300:H1210–H1221.57. Godoy AS, Chung I, Montecinos VP, Buttyan R, Johnson CS, Smith GJ. Role of androgen and vitamin D receptors in endothelial cells from benign and malignant human prostate. Am J Physiol Endocrinol Metab. 2013; 304:E1131–E1139.58. Ong PJ, Patrizi G, Chong WC, Webb CM, Hayward CS, Collins P. Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. Am J Cardiol. 2000; 85:269–272.59. Raitakari OT, Celermajer DS. Flow-mediated dilatation. Br J Clin Pharmacol. 2000; 50:397–404.60. Tarutani Y, Matsumoto T, Takashima H, Yamane T, Horie M. Brachial artery flow-mediated vasodilation is correlated with coronary vasomotor and fibrinolytic responses induced by bradykinin. Hypertens Res. 2005; 28:59–66.61. Kang SM, Jang Y, Kim Ji, Chung N, Cho SY, Chae JS, et al. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol. 2002; 89:862–864.62. Empen K, Lorbeer R, Dorr M, Haring R, Nauck M, Glaser S, et al. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol. 2012; 32:481–486.63. Perusquía M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol. 2010; 298:H1301–H1307.64. Eisermann K, Broderick CJ, Bazarov A, Moazam MM, Fraizer GC. Androgen up-regulates vascular endothelial growth factor expression in prostate cancer cells via an Sp1 binding site. Mol Cancer. 2013; 12:7.65. Fadini GP, Albiero M, Cignarella A, Bolego C, Pinna C, Boscaro E, et al. Effects of androgens on endothelial progenitor cells in vitro and in vivo. Clin Sci (Lond). 2009; 117:355–364.66. Foresta C, Zuccarello D, De Toni L, Garolla A, Caretta N, Ferlin A. Androgens stimulate endothelial progenitor cells through an androgen receptor-mediated pathway. Clin Endocrinol (Oxf). 2008; 68:284–289.67. Di Mambro A, Ferlin A, De Toni L, Selice R, Caretta N, Foresta C. Endothelial progenitor cells as a new cardiovascular risk factor in Klinefelter's syndrome. Mol Hum Reprod. 2010; 16:411–417.68. Foresta C, Caretta N, Lana A, Cabrelle A, Palu G, Ferlin A. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 2005; 17:288–290.69. Liao CH, Wu YN, Lin FY, Tsai WK, Liu SP, Chiang HS. Testosterone replacement therapy can increase circulating endothelial progenitor cell number in men with late onset hypogonadism. Andrology. 2013; 1:563–569.70. Cakir E, Ozcan O, Yaman H, Akgul EO, Bilgi C, Erbil MK, et al. Elevated plasma concentration of asymmetric dimethylarginine that is reduced by single dose testosterone administration in idiopathic hypogonadotropic hypogonadism patients. J Clin Endocrinol Metab. 2005; 90:1651–1654.71. Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001; 108:391–397.72. Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow: derived endothelial progenitor cells. J Clin Invest. 2001; 108:399–405.73. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005; 26:833–876.74. Garcia-Cruz E, Leibar-Tamayo A, Romero J, Piqueras M, Luque P, Cardenosa O, et al. Metabolic syndrome in men with low testosterone levels: relationship with cardiovascular risk factors and comorbidities and with erectile dysfunction. J Sex Med. 2013; 10:2529–2538.75. Camacho EM, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, Lee DM, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013; 168:445–455.76. Shi Z, Araujo AB, Martin S, O'Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013; 98:3289–3297.77. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013; 217:R25–R45.78. Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013; 9:479–493.79. Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med. 2011; 124:578–587.80. Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003; 107:3059–3065.81. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011; 96:3007–3019.82. Yassin AA, Saad F, Traish A. Testosterone undecanoate restores erectile function in a subset of patients with venous leakage: a series of case reports. J Sex Med. 2006; 3:727–735.83. Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994; 151:54–61.84. Zitzmann M, Brune M, Nieschlag E. Vascular reactivity in hypogonadal men is reduced by androgen substitution. J Clin Endocrinol Metab. 2002; 87:5030–5037.85. Morgentaler A, Sigman M, Miner M. Erectile dysfunction: a precursor to cardiovascular disease. Sex Reprod Menopause. 2009; 7:26–32.86. Bocchio M, Desideri G, Scarpelli P, Necozione S, Properzi G, Spartera C, et al. Endothelial cell activation in men with erectile dysfunction without cardiovascular risk factors and overt vascular damage. J Urol. 2004; 171:1601–1604.87. Ribeiro F, Ribeiro IP, Alves AJ, do Ceu Monteiro M, Oliveira NL, Oliveira J, et al. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: a systematic review. Am J Phys Med Rehabil. 2013; 92:1020–1030.88. Goglia L, Tosi V, Sanchez AM, Flamini MI, Fu XD, Zullino S, et al. Endothelial regulation of eNOS, PAI-1 and t-PA by testosterone and dihydrotestosterone in vitro and in vivo. Mol Hum Reprod. 2010; 16:761–769.89. Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, et al. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology. 2010; 151:1822–1828.90. Hirase T, Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am J Physiol Heart Circ Physiol. 2012; 302:H499–H505.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stem Cell Therapy for Erectile Dysfunction
- CD34 Expression in Pyogenic Granuloma
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Endothelial Progenitor Cells' Classification and Application in Neurological Diseases
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells