Korean J Urol.
2012 May;53(5):360-367.
Taxol and Taurine Protect the Renal Tissue of Rats after Unilateral Ureteral Obstruction: A Stereological Survey
- Affiliations
-
- 1Histomorphometry and Stereology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. noora@sums.ac.ir
Abstract
- PURPOSE
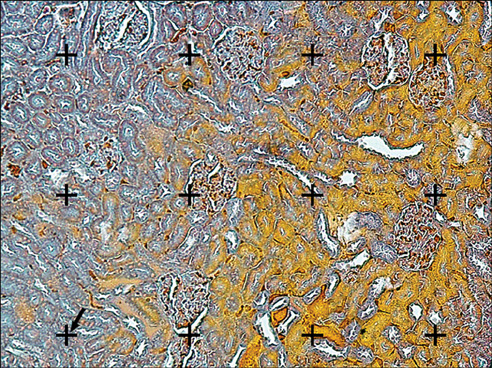
Blockage of the urinary tract induces changes in renal structure including tubular dilatation or atrophy, tubular cell death, inflammatory processes, and progressive interstitial fibrosis with the loss of renal parenchyma. The present study was conducted to survey the protective effects of Taxol and taurine on the renal structure after unilateral ureteral obstruction (UUO).
MATERIALS AND METHODS
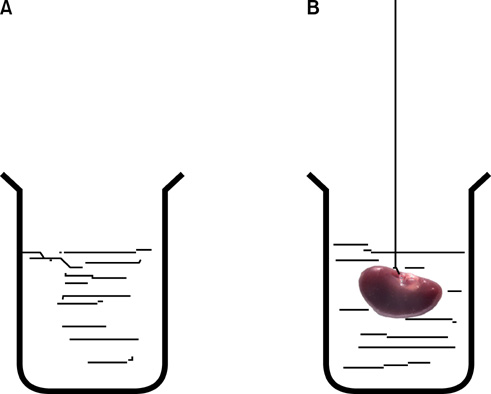
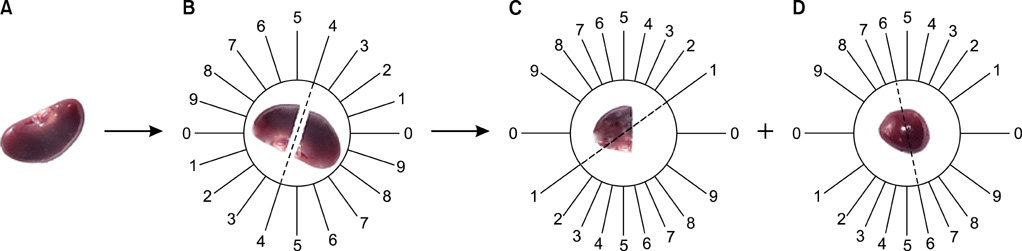
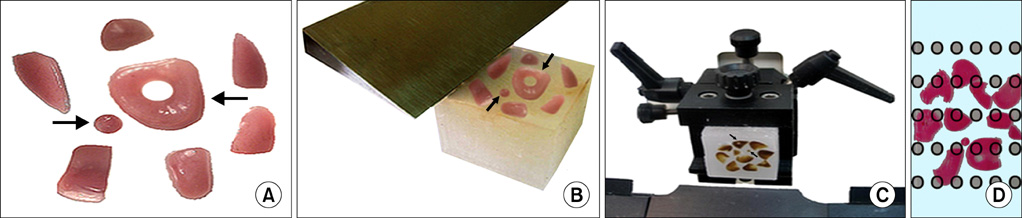
UUO was induced in three groups of rats (n=6) who then received distilled water, Taxol (0.3 mg/kg/d), or taurine (7.5 mg/kg/d). Stereological methods were used to gather quantitative as well as comparative data.
RESULTS
Less than -8% of the volume of the glomeruli, proximal convoluted tubules (PCT), distal convoluted tubules (DCT), Henle's loop, and collecting ducts were preserved after UUO. After treatment of the UUO rats with Taxol, between -32% and 88% of the parameters mentioned above remained intact, and after treatment of the UUO rats with taurine, between -16% and 46% of the parameters remained intact (p<0.01). Compared with the untreated UUO animals, the volume of necrotic and fibrotic tissues decreased -53% and -63% in the UUO rats treated with Taxol and taurine, respectively (p<0.01). Less than -3% of the lengths of the renal tubules (PCT, DCT, Henle's loop, and collecting) were preserved in the UUO rats. After treatment with Taxol and taurine, -61% to 70% and -43% to 53% of the length of the renal tubules were preserved, respectively (p<0.01).
CONCLUSIONS
Taurine and Taxol are effective in preventing some structural renal damage in a direct ureteral obstruction model. Taxol was more effective in renal protection.
Keyword
MeSH Terms
Figure
Reference
-
1. Sato S, Yamate J, Saito T, Hosokawa T, Saito S, Kurasaki M. Protective effect of taurine against renal interstitial fibrosis of rats induced by cisplatin. Naunyn Schmiedebergs Arch Pharmacol. 2002. 365:277–283.2. Chesney RW, Han X, Patters AB. Taurine and the renal system. J Biomed Sci. 2010. 17:Suppl 1. S4.3. Trachtman H, Futterweit S, Maesaka J, Ma C, Valderrama E, Fuchs A, et al. Taurine ameliorates chronic streptozocin-induced diabetic nephropathy in rats. Am J Physiol. 1995. 269(3 Pt 2):F429–F438.4. Cruz CI, Ruiz-Torres P, del Moral RG, Rodriguez-Puyol M, Rodríguez-Puyol D. Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am J Physiol Renal Physiol. 2000. 278:F122–F129.5. Marcinkiewicz J, Kurnyta M, Biedron R, Bobek M, Kontny E, Maślinski W. Anti-inflammatory effects of taurine derivatives (taurine chloramine, taurine bromamine, and taurolidine) are mediated by different mechanisms. Adv Exp Med Biol. 2006. 583:481–492.6. Odobasic D, Kitching AR, Semple TJ, Holdsworth SR. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol. 2007. 18:760–770.7. Suliman ME, Barany P, Filho JC, Lindholm B, Bergstrom J. Accumulation of taurine in patients with renal failure. Nephrol Dial Transplant. 2002. 17:528–529.8. Erdem A, Gundogan NU, Usubutun A, Kilinç K, Erdem SR, Kara A, et al. The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol Dial Transplant. 2000. 15:1175–1182.9. Sun L, Zhang D, Liu F, Xiang X, Ling G, Xiao L, et al. Low-dose paclitaxel ameliorates fibrosis in the remnant kidney model by down-regulating miR-192. J Pathol. 2011. 225:364–377.10. Zhang D, Sun L, Xian W, Liu F, Ling G, Xiao L, et al. Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab Invest. 2010. 90:436–447.11. Sommardahl CS, Woychik RP, Sweeney WE, Avner ED, Wilkinson JE. Efficacy of taxol in the orpk mouse model of polycystic kidney disease. Pediatr Nephrol. 1997. 11:728–733.12. Zhou J, Zhong DW, Wang QW, Miao XY, Xu XD. Paclitaxel ameliorates fibrosis in hepatic stellate cells via inhibition of TGF-beta/Smad activity. World J Gastroenterol. 2010. 16:3330–3334.13. Hyde DM, Tyler NK, Plopper CG. Morphometry of the respiratory tract: avoiding the sampling, size, orientation, and reference traps. Toxicol Pathol. 2007. 35:41–48.14. Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970. 26:57–60.15. Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999. 10:1100–1123.16. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988. 96:379–394.17. Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988. 96:857–881.18. Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol. 2008. 75:2214–2223.19. Guerrero-Beltran CE, Calderon-Oliver M, Tapia E, Medina-Campos ON, Sanchez-Gonzalez DJ, Martínez-Martinez CM, et al. Sulforaphane protects against cisplatin-induced nephrotoxicity. Toxicol Lett. 2010. 192:278–285.20. Mostafavi-Pour Z, Zal F, Monabati A, Vessal M. Protective effects of a combination of quercetin and vitamin E against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Hepatol Res. 2008. 38:385–392.21. Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Perez-Barriocanal F, Morales AI, Lopez-Novoa JM. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol Dial Transplant. 2011. 26:3484–3495.22. Yousef MI, Omar SA, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010. 48:3246–3261.23. Guerrero-Beltran CE, Mukhopadhyay P, Horvath B, Rajesh M, Tapia E, Garcia-Torres I, et al. Sulforaphane, a natural constituent of broccoli, prevents cell death and inflammation in nephropathy. J Nutr Biochem. 2012. 23:494–500.24. Koz OG, Ozhuy S, Tezel GG, Karaman N, Unlu N, Yarangumeli A, et al. The effect of paclitaxel on conjunctival wound healing: a pilot study. J Glaucoma. 2007. 16:610–615.25. Devi SL, Viswanathan P, Anuradha CV. Regression of liver fibrosis by taurine in rats fed alcohol: effects on collagen accumulation, selected cytokines and stellate cell activation. Eur J Pharmacol. 2010. 647:161–170.26. Devi SL, Anuradha CV. Oxidative and nitrosative stress in experimental rat liver fibrosis: Protective effect of taurine. Environ Toxicol Pharmacol. 2010. 29:104–110.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Application of the Partial Unilateral Ureteral Obstruction in Neonatal Rat Model
- Expression of Heat Shock Protein 70 in Ipsilateral Rat Kidney with Unilateral Partial Ureteral Obstruction
- Significance of Renal Resistive Index in Children with Unilateral Ureteral Obstruction
- Renal Expression of an Ammonia Transporter in Rats with a Unilateral Ureteral Obstruction
- A radiological study of recovery from hydronephrosis by ureteral ligation