Pediatr Gastroenterol Hepatol Nutr.
2012 Dec;15(4):237-242.
Efficacy of Proton Pump Inhibitor-based Triple Therapy and Bismuth-based Quadruple Therapy for Helicobacter pylori Eradication in Korean Children
- Affiliations
-
- 1Department of Pediatrics, Kangwon National University Hospital, Chuncheon, Korea.
- 2Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea. hryang@snubh.org
- 3Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
The aim of this study was to assess and compare the efficacies of proton pump inhibitor-based triple therapy and bismuth-based quadruple therapy as first-line treatments for Helicobacter pylori eradication in Korean children.
METHODS
We retrospectively reviewed the data of children who had been diagnosed with H. pylori infection at the Seoul National University Bundang Hospital from March 2004 to August 2012. The patients were randomly assigned to receive either triple therapy consisting of omeprazole, amoxicillin, and clarithromycin for 2 weeks (OAC group) or quadruple therapy comprising omeprazole, amoxicillin, metronidazole, and bismuth salts for 1 week (OAMB group). The patients were evaluated for eradication of H. pylori infection at 4 weeks after the completion of the treatment.
RESULTS
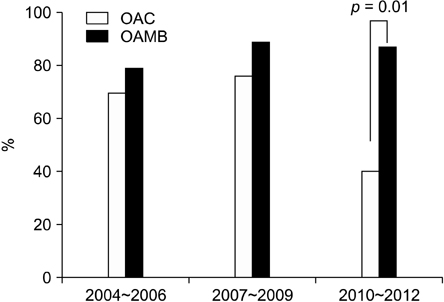
Of the 129 children enrolled in this study, 118 (91.5%) were included in the final analysis. The eradication rates in OAC and OAMB groups were 67.7% (42/62) and 83.9% (47/56), respectively, which were significantly different between the 2 treatment groups (p=0.041). The eradication rates in the OAMB group during the periods 2004-2006, 2007-2009, and 2010-2012 were superior to those in the OAC group.
CONCLUSION
This study indicated that the 1-week bismuth-based quadruple therapy, compared with the standard 2-week triple therapy, was significantly more successful in eradicating H. pylori infection in Korean children.
Keyword
MeSH Terms
Figure
Reference
-
1. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002. 347:1175–1186.2. Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/Florence Consensus Report. Gut. 2012. 61:646–664.
Article3. Gold BD, Colletti RB, Abbott M, Czinn SJ, Elitsur Y, Hassall E, et al. North American Society for Pediatric Gastroenterology and Nutrition. Helicobacter pylori infection in children: recommendations for diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2000. 31:490–497.
Article4. Kim SY, Jung SW. Helicobacter pylori eradication therapy in Korea. Korean J Gastroenterol. 2011. 58:67–73.5. Zullo A, Hassan C, Ridola L, De Francesco V, Vaira D. Standard triple and sequential therapies for Helicobacter pylori eradication: An update. Eur J Intern Med. 2012. [Epub ahead of print].6. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010. 59:1143–1153.
Article7. Kalach N, Benhamou PH, Campeotto F, Bergeret M, Dupont C, Raymond J. Clarithromycin resistance and eradication of Helicobacter pylori in children. Antimicrob Agents Chemother. 2001. 45:2134–2135.
Article8. Yang HR, Seo JK. Diagnostic accuracy of the C-urea breath test in children: adjustment of the cut-off value according to age. J Gastroenterol Hepatol. 2005. 20:264–269.
Article9. Koletzko S, Jones NL, Goodman KJ, Gold B, Rowland M, Cadranel S, et al. H pylori Working Groups of ESPGHAN and NASPGHAN. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011. 53:230–243.
Article10. Choi IK, Lee SY, Chung KS. Effect of one- or two-week triple therapy with omeprazole, amoxicillin, and clarithromycin on eradication of Helicobacter pylori infection in children. Korean J Pediatr Gastroenterol Nutr. 2002. 5:19–25.
Article11. Choi J, Jang JY, Kim JS, Park HY, Choe YH, Kim KM. Efficacy of two triple eradication regimens in children with Helicobacter pylori infection. J Korean Med Sci. 2006. 21:1037–1040.
Article12. Oderda G, Shcherbakov P, Bontems P, Urruzuno P, Romano C, Gottrand F, et al. European Pediatric Task Force on Helicobacter pylori. Results from the pediatric European register for treatment of Helicobacter pylori (PERTH). Helicobacter. 2007. 12:150–156.
Article13. Koletzko S, Richy F, Bontems P, Crone J, Kalach N, Monteiro ML, et al. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut. 2006. 55:1711–1716.
Article14. Hwang TJ, Kim N, Kim HB, Lee BH, Nam RH, Park JH, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010. 44:536–543.
Article15. Kim YM, Lee YJ, Oh SH, Sung H, Kim MN, Kim KM. Antimicrobial resistance of Helicobacter pylori isolated from Korean children. Korean J Pediatr Gastroenterol Nutr. 2011. 14:45–51.
Article16. Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol. 2008. 14:7361–7370.
Article17. Ertem D. Clinical practice: Helicobacter pylori infection in childhood. Eur J Pediatr. 2012. [Epub ahead of print].18. Kim JM. Antibiotic resistance of Helicobacter pylori isolated from Korean patients. Korean J Gastroenterol. 2006. 47:337–349.19. Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003. 98:562–567.
Article20. Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002. 123:1763–1769.
Article21. Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS. Korean College of Helicobacter and Upper Gastrointestinal Research. Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009. 54:269–278.
Article22. Jang HJ, Choi MH, Kim YS, Seo YA, Baik KH, Baik IH, et al. Effectiveness of triple therapy and quadruple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2005. 46:368–372.23. Jo HJ, Lee DH, Kang SJ, Kim MN, Kim SH, Park JM, et al. Comparison of the efficacy of bismuth containing PPI-based quadruple therapy with PPI-based triple therapy only as first-line treatment for Helicobacter pylori infection. Korean J Gastrointest Endosc. 2008. 37:259–264.24. Bahremand S, Nematollahi LR, Fourutan H, Tirgari F, Nouripour S, Mir E, et al. Evaluation of triple and quadruple Helicobacter pylori eradication therapies in Iranian children: a randomized clinical trial. Eur J Gastroenterol Hepatol. 2006. 18:511–514.
Article25. Dehghani SM, Erjaee A, Imanieh MH, Haghighat M. Efficacy of the standard quadruple therapy versus triple therapies containing proton pump inhibitor plus amoxicillin and clarithromycin or amoxicillin-clavulanic acid and metronidazole for Helicobacter pylori eradication in children. Dig Dis Sci. 2009. 54:1720–1724.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eradication Rates of Bismuth-based Quadruple Therapy as a Second-line Treatment for Helicobacter pylori Infection
- Recent Trends of Helicobacter pylori Eradication Therapy: Focusing on First Line Treatment
- Rescue therapies for Helicobacter pylori infection after failure of proton pump inhibitor-based standard triple therapy
- Current Strategies for Eradication of Helicobacter pylori in Korea
- Current Trends of Helicobacter pylori Eradication in Korea