Nutr Res Pract.
2015 Dec;9(6):628-636. 10.4162/nrp.2015.9.6.628.
Estrogen deprivation and excess energy supply accelerate 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in C3H/HeN mice
- Affiliations
-
- 1Department of Food and Nutrition, Sookmyung Women's University, 100 Chungpa-ro 47-gil, Yongsan-gu, Seoul, 140-742, Korea. mksung@sm.ac.kr
- 2Department of Food Science and Nutrition, College of Natural Sciences, Hallym University, 39 Hallymdaehak-gil, Chuncheon, 200-702, Korea.
- KMID: 2313887
- DOI: http://doi.org/10.4162/nrp.2015.9.6.628
Abstract
- BACKGROUND/OBJECTIVES
Obesity is a risk factor of breast cancer in postmenopausal women. Estrogen deprivation has been suggested to cause alteration of lipid metabolism thereby creating a cellular microenvironment favoring tumor growth. The aim of this study is to investigate the effects of estrogen depletion in combination with excess energy supply on breast tumor development.
MATERIALS/METHODS
Ovariectomized (OVX) or sham-operated C3H/HeN mice at 4 wks were provided with either a normal diet or a high-fat diet (HD) for 16 weeks. Breast tumors were induced by administration of 7,12-dimethylbenz(a)anthracene once a week for six consecutive weeks.
RESULTS
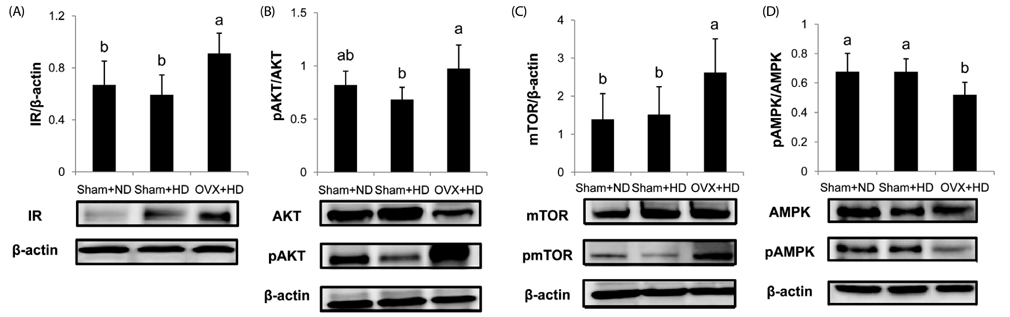
Study results showed higher serum concentrations of free fatty acids and insulin in the OVX+HD group compared to other groups. The average tumor volume was significantly larger in OVX+HD animals than in other groups. Expressions of mammary tumor insulin receptor and mammalian target of rapamycin proteins as well as the ratio of pAKT/AKT were significantly increased, while pAMPK/AMPK was decreased in OVX+HD animals compared to the sham-operated groups. Higher relative expression of liver fatty acid synthase mRNA was observed in OVX+HD mice compared with other groups.
CONCLUSIONS
These results suggest that excess energy supply affects the accelerated mammary tumor growth in estrogen deprived mice.
Keyword
MeSH Terms
-
Animals
Breast Neoplasms
Cellular Microenvironment
Diet
Diet, High-Fat
Estrogens*
Fatty Acids, Nonesterified
Female
Humans
Insulin
Lipid Metabolism
Liver
Mice*
Obesity
Postmenopause
Receptor, Insulin
Risk Factors
RNA, Messenger
TOR Serine-Threonine Kinases
Tumor Burden
Estrogens
Fatty Acids, Nonesterified
Insulin
RNA, Messenger
Receptor, Insulin
TOR Serine-Threonine Kinases
Figure
Reference
-
1. Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, Cleary MP. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010; 29:641–653.
Article2. Rohan TE, Heo M, Choi L, Datta M, Freudenheim JL, Kamensky V, Ochs-Balcom HM, Qi L, Thomson CA, Vitolins MZ, Wassertheil-Smoller S, Kabat GC. Body fat and breast cancer risk in postmenopausal women: a longitudinal study. J Cancer Epidemiol. 2013; 2013:754815.
Article3. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003; 348:1625–1638.
Article4. Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006; 296:193–201.
Article5. World Cancer Research Fund (GB). American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington D.C.: American Institute for Cancer Research;2007.6. World Cancer Research Fund International, Continuous Update Project (GB). Diet, Nutrition, Physical Activity, and the Breast Cancer Survivors. London: World Cancer Research Fund International;2014.7. Brennan SF, Woodside JV, Lunny PM, Cardwell CR, Cantwell MM. Dietary fat and breast cancer mortality: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. Forthcoming 2015.
Article8. Mourouti N, Kontogianni MD, Papavagelis C, Panagiotakos DB. Diet and breast cancer: a systematic review. Int J Food Sci Nutr. 2015; 66:1–42.
Article9. Demir B, Ozturkoglu E, Solaroglu A, Baskan B, Kandemir O, Karabulut E, Haberal A. The effects of estrogen therapy and estrogen combined with different androgenic progestins on carbohydrate and lipid metabolism in overweight-obese younger postmenopausal women. Gynecol Endocrinol. 2008; 24:347–353.
Article10. Jensen LB, Vestergaard P, Hermann AP, Gram J, Eiken P, Abrahamsen B, Brot C, Kolthoff N, Sørensen OH, Beck-Nielsen H, Nielsen SP, Charles P, Mosekilde L. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2003; 18:333–342.
Article11. Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood). 2004; 229:1127–1135.
Article12. D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005; 280:35983–35991.13. Field FJ, Born E, Murthy S, Mathur SN. Polyunsaturated fatty acids decrease the expression of sterol regulatory element-binding protein-1 in CaCo-2 cells: effect on fatty acid synthesis and triacylglycerol transport. Biochem J. 2002; 368:855–864.
Article14. Horton JD. Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem Soc Trans. 2002; 30:1091–1095.
Article15. Kuhl J, Hilding A, Ostenson CG, Grill V, Efendic S, Båvenholm P. Characterisation of subjects with early abnormalities of glucose tolerance in the Stockholm Diabetes Prevention Programme: the impact of sex and type 2 diabetes heredity. Diabetologia. 2005; 48:35–40.
Article16. Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009; 58:803–812.
Article17. Saengsirisuwan V, Pongseeda S, Prasannarong M, Vichaiwong K, Toskulkao C. Modulation of insulin resistance in ovariectomized rats by endurance exercise training and estrogen replacement. Metabolism. 2009; 58:38–47.
Article18. Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, Dahlman-Wright K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006; 20:1287–1299.
Article19. Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007; 8:395–408.
Article20. Jouyandeh Z, Nayebzadeh F, Qorbani M, Asadi M. Metabolic syndrome and menopause. J Diabetes Metab Disord. 2013; 12:1.
Article21. Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008; 114:23–37.
Article22. Kim HJ, Lee HO, Min DB. Effects and prooxidant mechanisms of oxidized alpha-tocopherol on the oxidative stability of soybean oil. J Food Sci. 2007; 72:C223–C230.23. Hong J, Holcomb VB, Kushiro K, Núñez NP. Estrogen inhibits the effects of obesity and alcohol on mammary tumors and fatty liver. Int J Oncol. 2011; 39:1443–1453.
Article24. Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001; 411:355–365.
Article25. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.
Article26. Park SY, Kim JS, Seo YR, Sung MK. Effects of diet-induced obesity on colitis-associated colon tumor formation in A/J mice. Int J Obes (Lond). 2012; 36:273–280.
Article27. Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer. 2003; 45:1–16.
Article28. Healy LA, Ryan AM, Carroll P, Ennis D, Crowley V, Boyle T, Kennedy MJ, Connolly E, Reynolds JV. Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol (R Coll Radiol). 2010; 22:281–288.
Article29. Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol. 2009; 587:5031–5037.
Article30. Hong J, Stubbins RE, Smith RR, Harvey AE, Núñez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 2009; 8:11.
Article31. Hakkak R, MacLeod S, Shaaf S, Holley AW, Simpson P, Fuchs G, Jo CH, Kieber-Emmons T, Korourian S. Obesity increases the incidence of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in an ovariectomized Zucker rat model. Int J Oncol. 2007; 30:557–563.
Article32. Sylvester PW, Ip C, Ip MM. Effects of high dietary fat on the growth and development of ovarian-independent carcinogen-induced mammary tumors in rats. Cancer Res. 1986; 46:763–769.33. Costa I, Solanas M, Escrich E. Histopathologic characterization of mammary neoplastic lesions induced with 7,12 dimethylbenz (alpha)anthracene in the rat: a comparative analysis with human breast tumors. Arch Pathol Lab Med. 2002; 126:915–927.
Article34. Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. 2008; 1241:1–6.
Article35. Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007; 354:45–49.
Article36. Lim DW, Lee Y, Kim YT. Preventive effects of Citrus unshiu peel extracts on bone and lipid metabolism in OVX rats. Molecules. 2014; 19:783–794.
Article37. Choi JS, Koh IU, Song J. Genistein reduced insulin resistance index through modulating lipid metabolism in ovariectomized rats. Nutr Res. 2012; 32:844–855.
Article38. Lane HW, Keith RE, Strahan S, White MT. The effect of diet, exercise and 7,12-dimethylbenz(a)anthracene on food intake, body composition and carcass energy levels in virgin female BALB/c mice. J Nutr. 1991; 121:1876–1882.
Article39. Ojeswi BK, Khoobchandani M, Hazra DK, Srivastava MM. Protective effect of Thuja occidentalis against DMBA-induced breast cancer with reference to oxidative stress. Hum Exp Toxicol. 2010; 29:369–375.
Article40. Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani AR. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 1997; 82:414–417.
Article41. Asp ML, Tian M, Wendel AA, Belury MA. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer. 2010; 126:756–763.
Article42. Suba Z. Circulatory estrogen level protects against breast cancer in obese women. Recent Pat Anticancer Drug Discov. 2013; 8:154–167.
Article43. Koricanac G, Milosavljevic T, Stojiljkovic M, Zakula Z, Ribarac-Stepic N, Isenovic ER. Insulin signaling in the liver and uterus of ovariectomized rats treated with estradiol. J Steroid Biochem Mol Biol. 2008; 108:109–116.
Article44. Swislocki A, Burgie ES, Rodnick KJ. Effects of ovariectomy on indices of insulin resistance, hypertension, and cardiac energy metabolism in middle-aged spontaneously hypertensive rats (SHR). Horm Metab Res. 2002; 34:516–522.
Article45. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003; 88:2404–2411.
Article46. Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002; 23:90–119.
Article47. Gu JW, Young E, Patterson SG, Makey KL, Wells J, Huang M, Tucker KB, Miele L. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol Ther. 2011; 11:910–917.
Article48. Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, Barrett JC, Hursting SD. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008; 60:534–541.
Article49. Asselin J, Labrie F. Effects of estradiol and prolactin on steroid receptor levels in 7,12-dimethylbenz(a)anthracene-induced mammary tumors and uterus in the rat. J Steroid Biochem. 1978; 9:1079–1082.
Article50. Sasaki GH, Leung BS. On the mechanism of hormone action in 7,12 dimethylbenz(a)anthracene-induced mammary tumor. I. Prolactin and progesterone effects on estrogen receptor in vitro. Cancer. 1975; 35:645–651.
Article51. Dao TL. The role of ovarian hormones in initiating the induction of mammary cancer in rats by polynuclear hydrocarbons. Cancer Res. 1962; 22:973–981.52. Rose DP, Gilhooly EM, Nixon DW. Adverse effects of obesity on breast cancer prognosis, and the biological actions of leptin (review). Int J Oncol. 2002; 21:1285–1292.
Article53. Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001; 92:720–729.
Article54. Belfiore A, Frasca F. IGF and insulin receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008; 13:381–406.
Article55. Wysocki PJ, Wierusz-Wysocka B. Obesity, hyperinsulinemia and breast cancer: novel targets and a novel role for metformin. Expert Rev Mol Diagn. 2010; 10:509–519.
Article56. Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002; 1:707–717.57. Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J, Guo X, Guo T, Botchlett R, Qi T, Pei Y, Zheng J, Xu Y, An X, Chen L, Chen L, Li Q, Xiao X, Huo Y, Wu C. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One. 2014; 9:e91111.
Article58. Wohlers LM, Sweeney SM, Ward CW, Lovering RM, Spangenburg EE. Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activated protein kinases in skeletal muscle after ovariectomy. J Cell Biochem. 2009; 107:171–178.
Article59. Tiainen M, Vaahtomeri K, Ylikorkala A, Mäkelä TP. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1). Hum Mol Genet. 2002; 11:1497–1504.
Article60. Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005; 18:283–293.
Article61. Chapuis N, Tamburini J, Green AS, Willems L, Bardet V, Park S, Lacombe C, Mayeux P, Bouscary D. Perspectives on inhibiting mTOR as a future treatment strategy for hematological malignancies. Leukemia. 2010; 24:1686–1699.
Article62. Kim JY, Jo KJ, Kim BJ, Baik HW, Lee SK. 17beta-estradiol induces an interaction between adenosine monophosphate-activated protein kinase and the insulin signaling pathway in 3T3-L1 adipocytes. Int J Mol Med. 2012; 30:979–985.
Article63. McInnes KJ, Brown KA, Hunger NI, Simpson ER. Regulation of LKB1 expression by sex hormones in adipocytes. Int J Obes (Lond). 2012; 36:982–985.
Article64. Kim JY, Jo KJ, Kim OS, Kim BJ, Kang DW, Lee KH, Baik HW, Han MS, Lee SK. Parenteral 17beta-estradiol decreases fasting blood glucose levels in non-obese mice with short-term ovariectomy. Life Sci. 2010; 87:358–366.
Article65. Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther. 2003; 2:S169–S177.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Compensatory role of C3 convertase on the strain difference for C3 protein expression in FVB/N, C3H/HeN and C57BL/6N mice
- Growth and development of Gymnophalloides seoi in immunocompetent and immunosuppressed C3H/HeN mice
- Expression of Cyclin D1, Cyclin E, p21Cip1 and p27Kip1 in Chemically Induced Rat Mammary Tumor Treated with Tamoxifen and Transforming Growth Factor-1
- Susceptibility of several strains of mice to Echinostoma hortense infection
- Tumor associated proteins of rat skin tumor induced by 7,12-dimethylbenz[a]anthracene