Nutr Res Pract.
2014 Apr;8(2):132-137.
Induction of apoptosis by a hexane extract of aged black garlic in the human leukemic U937 cells
- Affiliations
-
- 1Department of Molecular Biology, College of Natural Sciences, Dongeui University, Busan 614-714, Korea.
- 2Department of Horticultural Bioscience, College of Natural Resource and Life Sciences, Pusan National University, Gyeongnam 627-706, Korea.
- 3Duksan B&F Co. LTD., Busan 614-853, Korea.
- 4Laboratory of Immunobiology, Department of Marine Life Sciences, Jeju National University, Jeju 690-756, Korea.
- 5Anti-Aging Research Center & Blue-Bio Industry RIC, Dongeui University, Busan 614-714, Korea. choiyh@deu.ac.kr
- 6Department of Life Science and Biotechnology, College of Natural Science, Dongeui University, Busan 614-714, Korea.
- 7Department of Biochemistry, Dongeui University College of Oriental Medicine 52-57, Yangjeong-ro, Busanjin, Busan 614-052, Korea.
Abstract
- BACKGROUND/OBJECTIVES
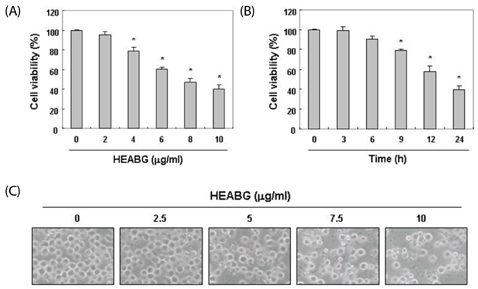
In this study, the apoptogenic activity and mechanisms of cell death induced by hexane extract of aged black garlic (HEABG) were investigated in human leukemic U937 cells.
MATERIALS/METHODS
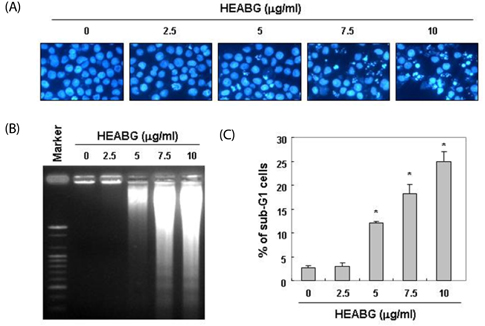
Cytotoxicity was evaluated by MTT (3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyl tetrazoliumbromide) assay. Apoptosis was detected using 4,6-diamidino-2-phenyllindile (DAPI) staining, agarose gel electrophoresis and flow cytometry. The protein levels were determined by Western blot analysis. Caspase activity was measured using a colorimetric assay.
RESULTS
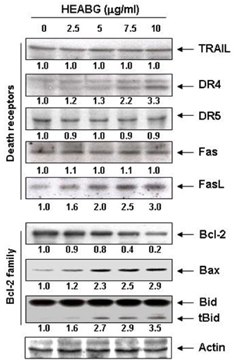
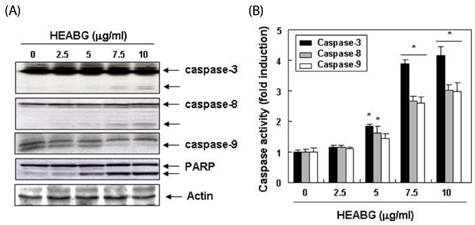
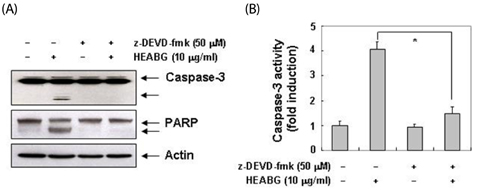
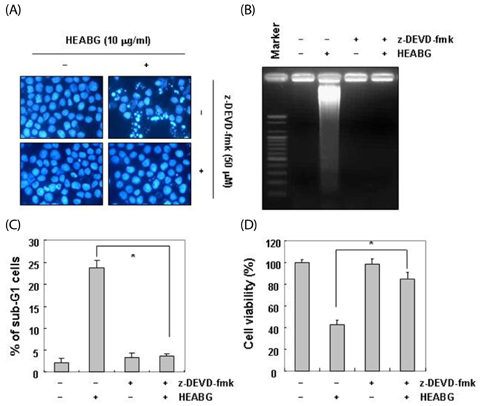
Exposure to HEABG was found to result in a concentration- and time-dependent growth inhibition by induction of apoptosis, which was associated with an up-regulation of death receptor 4 and Fas legend, and an increase in the ratio of Bax/Bcl-2 protein expression. Apoptosis-inducing concentrations of HEABG induced the activation of caspase-9, an initiator caspase of the mitochodrial mediated intrinsic pathway, and caspase-3, accompanied by proteolytic degradation of poly(ADP-ribose)-polymerase. HEABG also induced apoptosis via a death receptor mediated extrinsic pathway by caspase-8 activation, resulting in the truncation of Bid, and suggesting the existence of cross-talk between the extrinsic and intrinsic pathways. However, pre-treatment of U937 cells with the caspase-3 inhibitor, z-DEVD-fmk, significantly blocked the HEABG-induced apoptosis of these cells, and increased the survival rate of HEABG-treated cells, confirming that HEABG-induced apoptosis is mediated through activation of caspase cascade.
CONCLUSIONS
Based on the overall results, we suggest that HEABG reduces leukemic cell growth by inducing caspase-dependent apoptosis through both intrinsic and extrinsic pathways, implying its potential therapeutic value in the treatment of leukemia.
Keyword
MeSH Terms
Figure
Reference
-
1. Abramson N, Melton B. Leukocytosis: basics of clinical assessment. Am Fam Physician. 2000; 62:2053–2060.2. Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004; 80–97.
Article3. Voliotis D, Diehl V. Challenges in treating hematologic malignancies. Semin Oncol. 2002; 29:30–39.
Article4. Leone G, Fianchi L, Voso MT. Therapy-related myeloid neoplasms. Curr Opin Oncol. 2011; 23:672–680.
Article5. Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009; 9:501–507.
Article6. Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005; 4:139–163.
Article7. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516.
Article8. Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994; 371:346–347.
Article9. Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000; 6:513–519.
Article10. Kutuk O, Letai A. Regulation of Bcl-2 family proteins by posttranslational modifications. Curr Mol Med. 2008; 8:102–118.
Article11. Lee EW, Seo J, Jeong M, Lee S, Song J. The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep. 2012; 45:496–508.
Article12. Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998; 281:1305–1308.
Article13. Lee EN, Choi YW, Kim HK, Park JK, Kim HJ, Kim MJ, Lee HW, Kim KH, Bae SS, Kim BS, Yoon S. Chloroform extract of aged black garlic attenuates TNF-α-induced ROS generation, VCAM-1 expression, NF-κB activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phytother Res. 2011; 25:92–100.
Article14. Dolara P, Bigagli E, Collins A. Antioxidant vitamins and mineral supplementation, life span expansion and cancer incidence: a critical commentary. Eur J Nutr. 2012; 51:769–781.
Article15. Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets. 2003; 3:67–81.
Article16. Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature's protection against physiological threats. Crit Rev Food Sci Nutr. 2009; 49:538–551.
Article17. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994; 60:417–420.
Article18. Lee YM, Gweon OC, Seo YJ, Im J, Kang MJ, Kim MJ, Kim JI. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract. 2009; 3:156–161.
Article19. Kim HK, Choi YW, Lee EN, Park JK, Kim SG, Park DJ, Kim BS, Lim YT, Yoon S. 5-Hydroxymethylfurfural from black garlic extract prevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phytother Res. 2011; 25:965–974.
Article20. Jeong YY, Park HJ, Cho YW, Kim EJ, Kim GT, Mun YJ, Lee JD, Shin JH, Sung NJ, Kang D, Han J. Aged red garlic extract reduces cigarette smoke extract-induced cell death in human bronchial smooth muscle cells by increasing intracellular glutathione levels. Phytother Res. 2012; 26:18–25.
Article21. Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid Med Cell Longev. 2012; 2012:907162.
Article22. Wang X, Jiao F, Wang QW, Wang J, Yang K, Hu RR, Liu HC, Wang HY, Wang YS. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol Med Rep. 2012; 5:66–72.23. Kim KH, Park JK, Choi YW, Kim YH, Lee EN, Lee JR, Kim HS, Baek SY, Kim BS, Lee KS, Yoon S. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM-1 in TNF-α-activated human endometrial stromal cells. Int J Mol Med. 2013; 32:67–78.
Article24. Zhao H, Yang Z, Wang X, Zhang X, Wang M, Wang Y, Mei Q, Wang Z. Triptolide inhibits ovarian cancer cell invasion by repression of matrix metalloproteinase 7 and 19 and upregulation of E-cadherin. Exp Mol Med. 2012; 44:633–641.
Article25. Li Z, Gao Q. Induction of apoptosis in HT-29 cells by quercetin through mitochondria-mediated apoptotic pathway. Anim Cells Syst. 2013; 17:147–153.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HS-1200 Overcomes the Resistance Conferred by Bcl-2 in Human Leukemic U937 Cells
- Effects of allyl sulfur compounds and garlic extract on the expression of Bcl-2, Bax, and p53 in non small cell lung cancer cell lines
- Induction of Apoptosis Scutellaria baicalensis Georgi Root Extract by Inactivation of the Phosphatidyl Inositol 3-kinase/Akt Signaling Pathway in Human Leukemia U937 Cells
- The Apoptotic Effect of the Hexane Extract of Rheum undulatum L. in Oral Cancer Cells through the Down-regulation of Specificity Protein 1 and Survivin
- Adriamycin Induces Apoptosis of Human Myeloid Leukemic U937 Cells via Activation of Caspase-3 and cJun-N Terminal Kinase1(JNK1)/Stress Activated Protein Kinase(SAPK)