Nutr Res Pract.
2013 Dec;7(6):460-465.
Acanthopanax koreanum Nakai modulates the immune response by inhibiting TLR 4-dependent cytokine production in rat model of endotoxic shock
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 52, Ewhayeodae-gil, Seodaemun-gu, Seoul 120-750, Korea. orank@ewha.ac.kr
- 2Department of Biomedical Laboratory Science, Eulji University, Seongnam, Gyeonggi 461-713, Korea.
- 3Jeju Biodiversity Research Institute, Jeju Technopark, Jeju 699-943, Korea.
Abstract
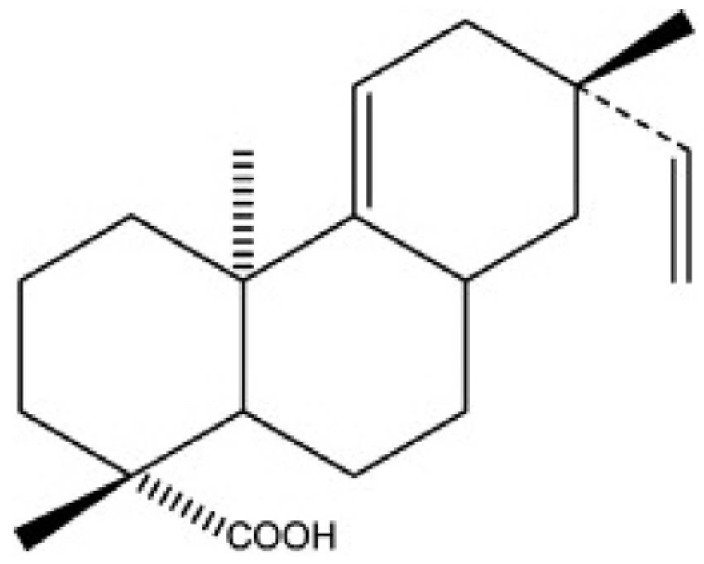
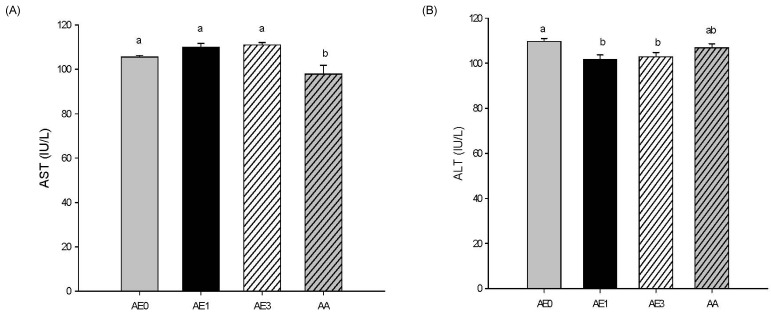
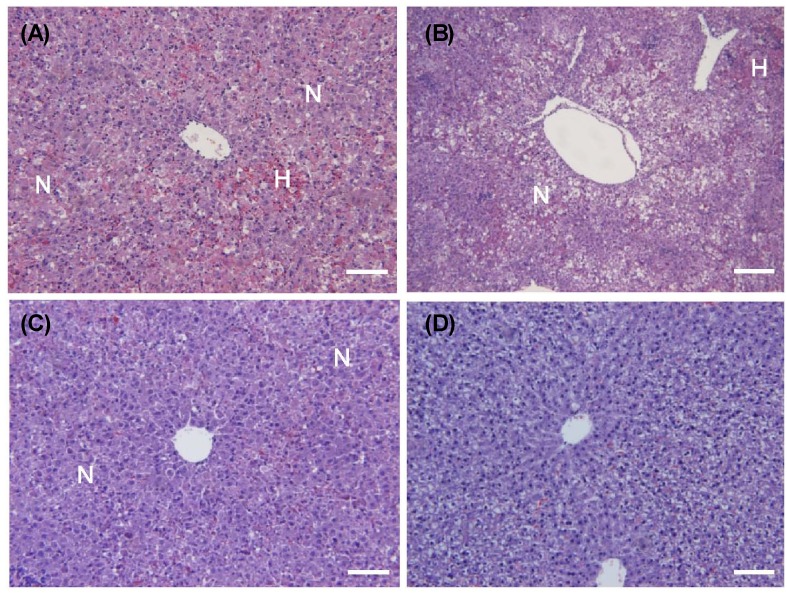
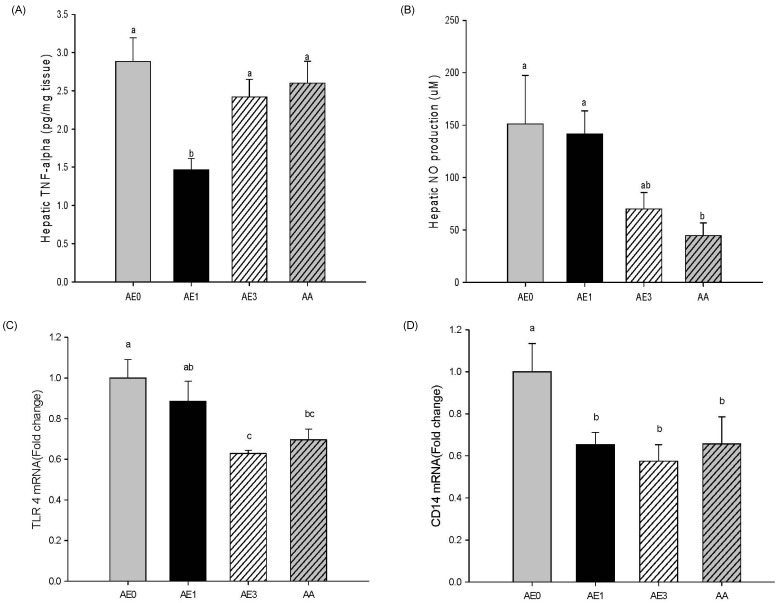
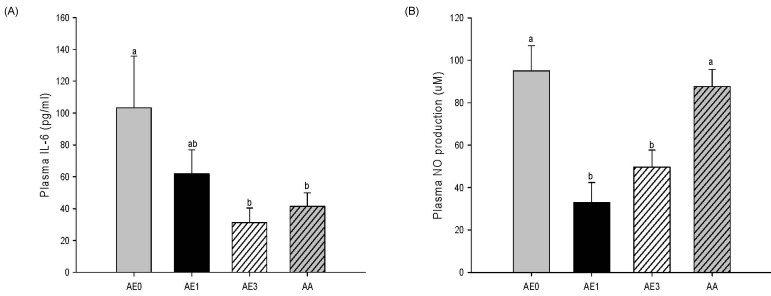
- The hepatoprotective activity of Acanthopanax koreanum Nakai extract (AE) was investigated against D-Galactosamine/Lipopolysaccharide (D-GalN/LPS)-induced liver failure rats compared with that of acanthoic acid (AA) isolated from AE. Although D-GalN/LPS (250 mg/kg body weight/10 microg/kg body weight, i.p.) induced hepatic damage, pretreatments with AE (1 and 3% AE/g day) and AA (0.037% AA, equivalent to 3% AE/g day) alleviated the hepatic damage. This effect was the result of a significant decrease in the activity of alanine transaminase. Concomitantly, both the nitric oxide and IL-6 levels in the plasma were significantly decreased by high-dose AE (AE3) treatment compared to the GalN/LPS control (AE0). This response resulted from the regulation of pro-inflammatory signaling via a decrease in TLR4 and CD14 mRNA levels in the liver. While a high degree of necrosis and hemorrhage were observed in the AE0, pretreatment with AE3 and AA reduced the extent of hepatocyte degeneration, necrosis, hemorrhage and inflammatory cell infiltrates compared to the AE0. In conclusion, these results suggest that especially high-dose AE are capable of alleviating D-GalN/LPS-induced hepatic injury by decreasing hepatic toxicity, thereby mitigating the TLR 4-dependent cytokine release. The anti-inflammatory effect of AE could be contributing to that of AA and AE is better than AA.
Keyword
MeSH Terms
-
Eleutherococcus*
Alanine Transaminase
Animals
Body Weight
Diterpenes
Hemorrhage
Hepatocytes
Inflammation
Interleukin-6
Liver
Liver Failure
Models, Animal*
Necrosis
Nitric Oxide
Plasma
Rats*
RNA, Messenger
Shock, Septic*
Toll-Like Receptor 4
Alanine Transaminase
Diterpenes
Interleukin-6
Nitric Oxide
RNA, Messenger
Toll-Like Receptor 4
Figure
Reference
-
1. Neihörster M, Inoue M, Wendel A. A link between extracellular reactive oxygen and endotoxin-induced release of tumour necrosis factor α in vivo. Biochem Pharmacol. 1992; 43:1151–1154. PMID: 1554388.
Article2. Mayer AM, Spitzer JA. Modulation of superoxide anion generation by manoalide, arachidonic acid and staurosporine in liver infiltrated neutrophils in a rat model of endotoxemia. J Pharmacol Exp Ther. 1993; 267:400–409. PMID: 8229768.3. Fiuza C, Suffredini AF. Human models of innate immunity: local and systemic inflammatory responses. J Endotoxin Res. 2001; 7:385–388. PMID: 11753208.
Article4. Ben Ari Z, Avlas O, Pappo O, Zilbermints V, Cheporko Y, Bachmetov L, Zemel R, Shainberg A, Sharon E, Grief F, Hochhauser E. Reduced hepatic injury in Toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell Physiol Biochem. 2012; 29:41–50. PMID: 22415073.
Article5. Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000; 164:5564–5574. PMID: 10820230.
Article6. Kang HS, Kim YH, Lee CS, Lee JJ, Choi I, Pyun KH. Suppression of interleukin-1 and tumor necrosis factor-α production by acanthoic acid, (-)-pimara-9(11),15-dien-19-oic acid, and it antifibrotic effects in vivo. Cell Immunol. 1996; 170:212–221. PMID: 8660820.7. Park EJ, Zhao YZ, Kim YH, Lee JJ, Sohn DH. Acanthoic acid from Acanthopanax koreanum protects against liver injury induced by tert-butyl hydroperoxide or carbon tetrachloride in vitro and in vivo. Planta Med. 2004; 70:321–327. PMID: 15095147.8. Nan JX, Jin XJ, Lian LH, Cai XF, Jiang YZ, Jin HR, Lee JJ. A diterpenoid acanthoic acid from Acanthopanax koreanum protects against D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Biol Pharm Bull. 2008; 31:738–742. PMID: 18379074.
Article9. Wu YL, Jiang YZ, Jin XJ, Lian LH, Piao JY, Wan Y, Jin HR, Joon Lee J, Nan JX. Acanthoic acid, a diterpene in Acanthopanax koreanum, protects acetaminophen-induced hepatic toxicity in mice. Phytomedicine. 2010; 17:475–479. PMID: 19836221.
Article10. Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009; 119:1858–1870. PMID: 19603542.
Article11. Qureshi ST, Gros P, Malo D. Host resistance to infection: genetic control of lipopolysaccharide responsiveness by TOLL-like receptor genes. Trends Genet. 1999; 15:291–294. PMID: 10431187.
Article12. Kitazawa T, Tsujimoto T, Kawaratani H, Fujimoto M, Fukui H. Expression of Toll-like receptor 4 in various organs in rats with D-galactosamine-induced acute hepatic failure. J Gastroenterol Hepatol. 2008; 23:e494–e498. PMID: 18070011.
Article14. Nakama T, Hirono S, Moriuchi A, Hasuike S, Nagata K, Hori T, Ido A, Hayashi K, Tsubouchi H. Etoposide prevents apoptosis in mouse liver with D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure resulting in reduction of lethality. Hepatology. 2001; 33:1441–1450. PMID: 11391533.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Mutagenic Properties of Two Lignans from Acanthopanax koreanum Nakai
- Protective effects of Acanthopanax koreanum Kakai extract against carbon tetrachloride-induced liver injury in Sprague-Dawley rats
- The Effect of Acanthopanax koreanum Extract on the Induction of Collagen Induced Arthritis for DBA/1J Mice
- Yield Analysis of Flavonoids in Acanthopanax divaricatus and A. koreanum Grown using Different Cultivation Methods
- Effects of an Opiate Receptor Antagonist Naloxone on Endotoxic Shock and Tumorigenesis