Nutr Res Pract.
2013 Aug;7(4):249-255.
Epigallocatechin gallate attenuates L-DOPA-induced apoptosis in rat PC12 cells
- Affiliations
-
- 1Department of Food and Nutrition, College of Natural Science, Chosun University, 301, Pilmun-daero, Dong-gu, Gwangju 501-759, Korea. leejj80@chosun.ac.kr
- 2College of Pharmacy, Chosun University, Gwangju 501-759, Korea.
- 3College of Pharmacy, Chungbuk National University, Cheongju, Chungbuk 361-763, Korea.
Abstract
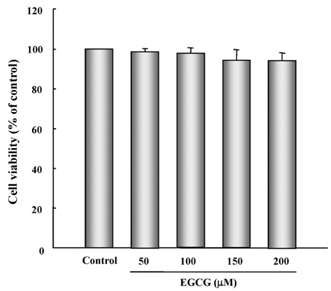
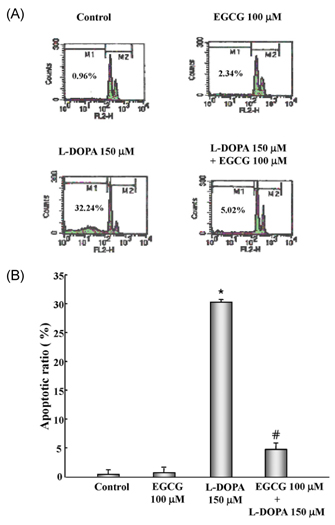
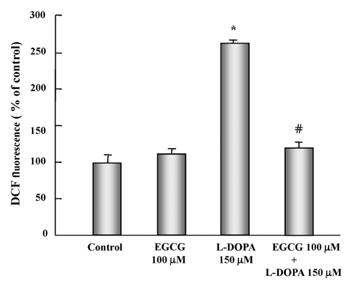
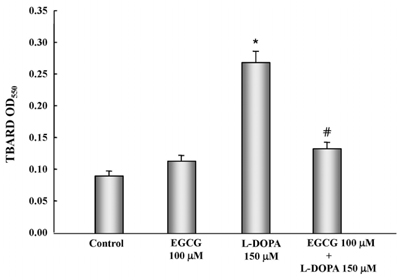
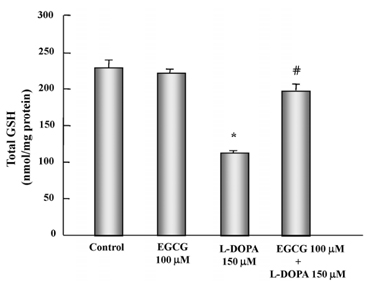
- In this study, the protective effects of EGCG on L-3,4-dihydroxyphenylalanine (L-DOPA)-induced oxidative cell death in catecholaminergic PC12 cells, the in vitro model of Parkinson's disease, were investigated. Treatment with L-DOPA at concentrations higher than 150 microM caused cytotoxicity in PC12 cells, as determined using the 3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and flow cytometry detection. The apoptotic ratio was similar in cells treated with 100 microM EGCG plus 150 microM L-DOPA (5.02%) and the control (0.96%) (P > 0.05), and was lower than that of cells treated with L-DOPA only (32.24%, P < 0.05). The generation level of ROS (% of control) in cells treated with EGCG plus L-DOPA was lower than that in cells treated with L-DOPA only (123.90% vs 272.32%, P < 0.05). The optical density in production of TBARS in cells treated with L-DOPA only was higher than that in the control (0.27 +/- 0.05 vs 0.08 +/- 0.04, P < 0.05), and in cells treated with EGCG only (0.14 +/- 0.02, P < 0.05), and EGCG plus L-DOPA (0.13 +/- 0.02, P < 0.05). The intracellular level of GSH in cells treated with EGCG plus L-DOPA was higher than that in cells treated with L-DOPA only (233.25 +/- 16.44 vs 119.23 +/- 10.25, P < 0.05). These results suggest that EGCG protects against L-DOPA-induced oxidative apoptosis in PC12 cells, and might be a potent neuroprotective agent.
Keyword
MeSH Terms
Figure
Reference
-
1. Agid Y. Parkinson's disease: pathophysiology. Lancet. 1991; 337:1321–1324.
Article2. Lee JJ, Kim YM, Yin SY, Park HD, Kang MH, Hong JT, Lee MK. Aggravation of L-DOPA-induced neurotoxicity by tetrahydropapaveroline in PC12 cells. Biochem Pharmacol. 2003; 66:1787–1795.
Article3. Migheli R, Godani C, Sciola L, Delogu MR, Serra PA, Zangani D, De Natale G, Miele E, Desole MS. Enhancing effect of manganese on L-DOPA-induced apoptosis in PC12 cells: role of oxidative stress. J Neurochem. 1999; 73:1155–1163.4. Spencer JP, Jenner P, Halliwell B. Superoxide-dependent depletion of reduced glutathione by L-DOPA and dopamine. Relevance to Parkinson's disease. Neuroreport. 1995; 6:1480–1484.
Article5. Hirsch EC. Biochemistry of Parkinson's disease with special reference to the dopaminergic systems. Mol Neurobiol. 1994; 9:135–142.
Article6. Mizuno Y, Ikebe S, Hattori N, Nakagawa-Hattori Y, Mochizuki H, Tanaka M, Ozawa T. Role of mitochondria in the etiology and pathogenesis of Parkinson's disease. Biochim Biophys Acta. 1995; 1271:265–274.
Article7. Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989; 52:381–389.
Article8. Chung KK, Dawson VL, Dawson TM. New insights into Parkinson's disease. J Neurol. 2003; 250:Suppl 3. III15–III24.
Article9. Heo HJ, Choi SJ, Choi SG, Shin DH, Lee JM, Lee CY. Effects of banana, orange, and apple on oxidative stress-induced neurotoxicity in PC12 cells. J Food Sci. 2008; 73:H28–H32.
Article10. Bastianetto S, Zheng WH, Quirion R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: involvement of its flavonoid constituents and protein kinase C. J Neurochem. 2000; 74:2268–2277.
Article11. Bordoni A, Hrelia S, Angeloni C, Giordano E, Guarnieri C, Caldarera CM, Biagi PL. Green tea protection of hypoxia/reoxygenation injury in cultured cardiac cells. J Nutr Biochem. 2002; 13:103–111.
Article12. Nie G, Jin C, Cao Y, Shen S, Zhao B. Distinct effects of tea catechins on 6-hydroxydopamine-induced apoptosis in PC12 cells. Arch Biochem Biophys. 2002; 397:84–90.
Article13. Song DU, Jung YD, Chay KO, Chung MA, Lee KH, Yang SY, Shin BA, Ahn BW. Effect of drinking green tea on age-associated accumulation of Maillard-type fluorescence and carbonyl groups in rat aortic and skin collagen. Arch Biochem Biophys. 2002; 397:424–429.
Article14. Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (-)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997; 57:4414–4419.15. Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta. 1996; 1304:210–222.
Article16. Guo Q, Zhao B, Shen S, Hou J, Hu J, Xin W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim Biophys Acta. 1999; 1427:13–23.
Article17. Jin CF, Shen SR Sr, Zhao BL. Different effects of five catechins on 6-hydroxydopamine-induced apoptosis in PC12 cells. J Agric Food Chem. 2001; 49:6033–6038.
Article18. Levites Y, Youdim MB, Maor G, Mandel S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappaB) activation and cell death by tea extracts in neuronal cultures. Biochem Pharmacol. 2002; 63:21–29.
Article19. Yoneda T, Hiramatsu M, Sakamoto M, Togasaki K, Komatsu M, Yamaguchi K. Antioxidant effects of "beta catechin". Biochem Mol Biol Int. 1995; 35:995–1008.20. Koh SH, Kim SH, Kwon H, Park Y, Kim KS, Song CW, Kim J, Kim MH, Yu HJ, Henkel JS, Jung HK. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Brain Res Mol Brain Res. 2003; 118:72–81.
Article21. Casarejos MJ, Solano RM, Menéndez J, Rodríguez-Navarro JA, Correa C, García de Yébenes J, Mena MA. Differential effects of L-DOPA on monoamine metabolism, cell survival and glutathione production in midbrain neuronal-enriched cultures from parkin knockout and wild-type mice. J Neurochem. 2005; 94:1005–1014.
Article22. Walkinshaw G, Waters CM. Induction of apoptosis in catecholaminergic PC12 cells by L-DOPA. Implications for the treatment of Parkinson's disease. J Clin Invest. 1995; 95:2458–2464.
Article23. Boyce S, Rupniak NM, Steventon MJ, Iversen SD. Nigrostriatal damage is required for induction of dyskinesias by L-DOPA in squirrel monkeys. Clin Neuropharmacol. 1990; 13:448–458.
Article24. Cheng N, Maeda T, Kume T, Kaneko S, Kochiyama H, Akaike A, Goshima Y, Misu Y. Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons. Brain Res. 1996; 743:278–283.
Article25. Basma AN, Morris EJ, Nicklas WJ, Geller HM. L-DOPA cytotoxicity to PC12 cells in culture is via its autoxidation. J Neurochem. 1995; 64:825–832.
Article26. Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976; 73:2424–2428.
Article27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article28. Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J Neurochem. 1995; 65:2016–2021.
Article29. Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990; 186:421–431.30. Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980; 106:207–212.
Article31. Raza H, John A. In vitro effects of tea polyphenols on redox metabolism, oxidative stress, and apoptosis in PC12 cells. Ann N Y Acad Sci. 2008; 1138:358–365.
Article32. Watanabe H, Kobayashi A, Yamamoto T, Suzuki S, Hayashi H, Yamazaki N. Alterations of human erythrocyte membrane fluidity by oxygen-derived free radicals and calcium. Free Radic Biol Med. 1990; 8:507–514.
Article33. Zeevalk GD, Bernard LP, Albers DS, Mirochnitchenko O, Nicklas WJ, Sonsalla PK. Energy stress-induced dopamine loss in glutathione peroxidase-overexpressing transgenic mice and in glutathione-depleted mesencephalic cultures. J Neurochem. 1997; 68:426–429.
Article34. Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem. 2001; 78:1073–1082.
Article35. Levites Y, Youdim MB, Maor G, Mandel S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappaB) activation and cell death by tea extracts in neuronal cultures. Biochem Pharmacol. 2002; 63:21–29.
Article36. Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. FASEB J. 2003; 17:952–954.37. Jeong JH, Kim HJ, Lee TJ, Kim MK, Park ES, Choi BS. Epigallocatechin 3-gallate attenuates neuronal damage induced by 3-hydroxykynurenine. Toxicology. 2004; 195:53–60.
Article38. Jung JY, Mo HC, Yang KH, Jeong YJ, Yoo HG, Choi NK, Oh WM, Oh HK, Kim SH, Lee JH, Kim HJ, Kim WJ. Inhibition by epigallocatechin gallate of CoCl2-induced apoptosis in rat PC12 cells. Life Sci. 2007; 80:1355–1363.
Article39. Inanami O, Watanabe Y, Syuto B, Nakano M, Tsuji M, Kuwabara M. Oral administration of (-)catechin protects against ischemia-reperfusion-induced neuronal death in the gerbil. Free Radic Res. 1998; 29:359–365.
Article40. Seyfried J, Soldner F, Schulz JB, Klockgether T, Kovar KA, Wullner U. Differential effects of L-buthionine sulfoximine and ethacrynic acid on glutathione levels and mitochondrial function in PC12 cells. Neurosci Lett. 1999; 264:1–4.
Article41. Wüllner U, Löschmann PA, Schulz JB, Schmid A, Dringen R, Eblen F, Turski L, Klockgether T. Glutathione depletion potentiates MPTP and MPP+ toxicity in nigral dopaminergic neurones. Neuroreport. 1996; 7:921–923.
Article42. Raza H, John A. Green tea polyphenol epigallocatechin-3-gallate differentially modulates oxidative stress in PC12 cell compartments. Toxicol Appl Pharmacol. 2005; 207:212–220.
Article43. Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002; 9:232–238.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Green tea polyphenol (–)-epigallocatechin-3-gallate prevents ultraviolet-induced apoptosis in PC12 cells
- PC12 Cell Apoptosis Mediated by Reactive Oxygen Species with Various Agents is Reduced Effectively by Flavonoid Antioxidants
- Inhibition of Nitric Oxide-induced Neuronal Apoptosis in PC12 Cells by Epigallocatechin Gallate
- Cytosine Arabinoside-Induced PC12 Cell Death Pathway
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts