Nutr Res Pract.
2012 Apr;6(2):113-119.
Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet
- Affiliations
-
- 1Department of Nutritional Sciences, University of Connecticut, 3624 Horsebarn Rd ext, Storrs, CT 06269, USA. maria-luz.fernandez@uconn.edu
Abstract
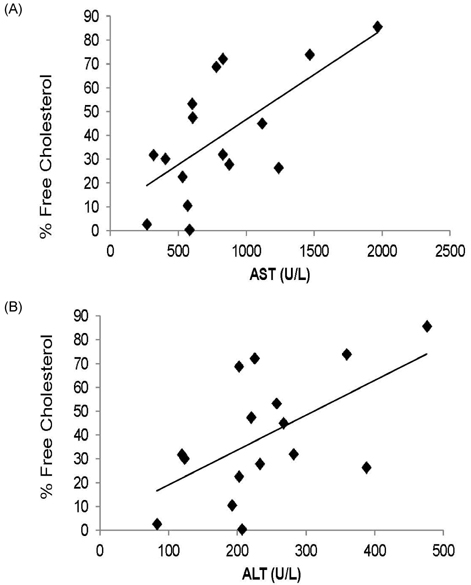
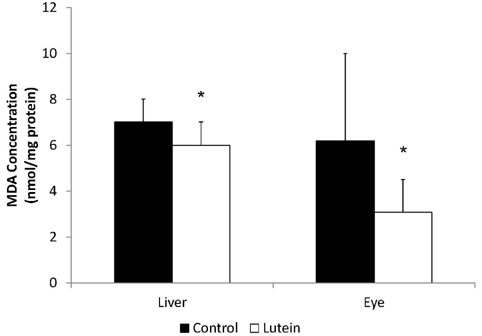
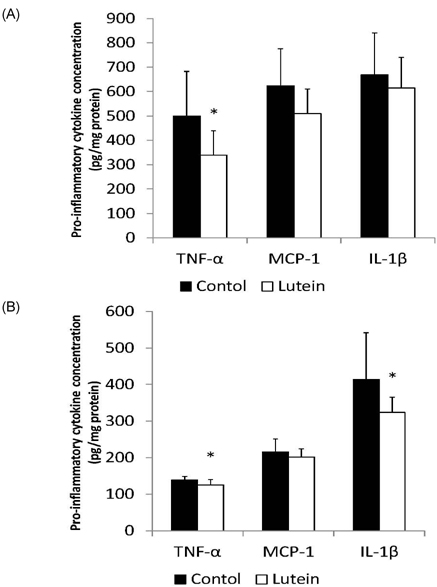
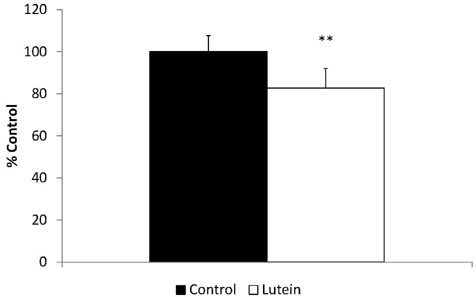
- Guinea pigs were fed a hypercholesterolemic diet (0.25 g/100 g cholesterol) and randomly allocated either to a Control group (n = 9) or to a Lutein (0.1 g/100 g) group (n = 10) for 12 weeks to evaluate oxidative stress and inflammation in both liver and eyes. Malondialdehyde (MDA) concentrations and inflammatory cytokines were measured as well as hepatic nuclear factor-kappaB (NF-kappaB) binding. Lutein concentrations were greater in eyes (P < 0.01) and liver (P < 0.001) in the Lutein group. All guinea pigs had high concentrations of hepatic cholesterol as well as high plasma ALT and AST levels indicative of liver injury. However, the Lutein group had 43% lower hepatic free cholesterol than the Controls (P < 0.05). Hepatic MDA and MDA in the eye were lower in the Lutein compared to the Control group (P < 0.05). Hepatic tumor necrosis factor-alpha was 32% lower in the Lutein group (P < 0.05). Lastly, the Lutein group presented lower NF-kappaB DNA binding activity than the Control group (P < 0.001). These results suggest that in the presence of high cholesterol, lutein exerts both antioxidant and anti-inflammatory effects, which can be explained by attenuated NF-kappaB DNA binding activity. Furthermore, results also suggest that lutein accumulates in the eyes of guinea pigs to protect against oxidative stress.
Keyword
MeSH Terms
Figure
Reference
-
1. Khachik F, Beecher GR, Smith JC Jr. Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J Cell Biochem Suppl. 1995. 22:236–246.
Article2. Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003. 23:171–201.
Article3. Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006. 51:137–152.
Article4. Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008. 14:194–198.
Article5. Dwyer JH, Navab M, Dwyer KM, Hassan K, Sun P, Shircore A, Hama-Levy S, Hough G, Wang X, Drake T, Merz CN, Fogelman AM. Oxygenated carotenoid lutein and progression of early atherosclerosis: the Los Angeles atherosclerosis study. Circulation. 2001. 103:2922–2927.
Article6. Dwyer JH, Paul-Labrador MJ, Fan J, Shircore AM, Merz CN, Dwyer KM. Progression of carotid intima-media thickness and plasma antioxidants: the Los Angeles Atherosclerosis Study. Arterioscler Thromb Vasc Biol. 2004. 24:313–319.
Article7. Sindhu ER, Firdous AP, Preethi KC, Kuttan R. Carotenoid lutein protects rats from paracetamol-, carbon tetrachloride- and ethanol-induced hepatic damage. J Pharm Pharmacol. 2010. 62:1054–1060.
Article8. Sindhu ER, Preethi KC, Kuttan R. Antioxidant activity of carotenoid lutein in vitro and in vivo. Indian J Exp Biol. 2010. 48:843–848.9. Jin XH, Ohgami K, Shiratori K, Suzuki Y, Hirano T, Koyama Y, Yoshida K, Ilieva I, Iseki K, Ohno S. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2006. 47:2562–2568.
Article10. Kim JH, Na HJ, Kim CK, Kim JY, Ha KS, Lee H, Chung HT, Kwon HJ, Kwon YG, Kim YM. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: role of H(2)O(2) in NF-kappaB activation. Free Radic Biol Med. 2008. 45:885–896.
Article11. Shanmugasundaram R, Selvaraj RK. Lutein supplementation alters inflammatory cytokine production and antioxidant status in F-line turkeys. Poult Sci. 2011. 90:971–976.
Article12. He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011. 21:159–168.
Article13. Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011. 8:108–118.
Article14. Robinson SM, Mann DA. Role of nuclear factor kappaB in liver health and disease. Clin Sci (Lond). 2010. 118:691–705.15. Fernandez ML. Guinea pigs as models for cholesterol and lipoprotein metabolism. J Nutr. 2001. 131:10–20.
Article16. Roy S, Vega-Lopez S, Fernandez ML. Gender and hormonal status affect the hypolipidemic mechanisms of dietary soluble fiber in guinea pigs. J Nutr. 2000. 130:600–607.
Article17. Torres-Gonzalez M, Shrestha S, Sharman M, Freake HC, Volek JS, Fernandez ML. Carbohydrate restriction alters hepatic cholesterol metabolism in guinea pigs fed a hypercholesterolemic diet. J Nutr. 2007. 137:2219–2223.
Article18. Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006. 87:1–16.
Article19. Kim JE, Leite JO, DeOgburn R, Smyth JA, Clark RM, Fernandez ML. A lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J Nutr. 2011. 141:1458–1463.
Article20. Fernandez ML, McNamara DJ. Regulation of cholesterol and lipoprotein metabolism in guinea pigs mediated by dietary fat quality and quantity. J Nutr. 1991. 121:934–943.
Article21. Clark RM, Herron KL, Waters D, Fernandez ML. Hypo- and hyperresponse to egg cholesterol predicts plasma lutein and beta-carotene concentrations in men and women. J Nutr. 2006. 136:601–607.
Article22. Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993. 26:39–42.
Article23. Schäffer MW, Sinha Roy S, Mukherjee S, Das SK. Identification of lutein, a dietary antioxidant carotenoid in guinea pig tissues. Biochem Biophys Res Commun. 2008. 374:378–381.
Article24. Lin EC, Fernandez ML, McNamara DJ. Dietary fat type and cholesterol quantity interact to affect cholesterol metabolism in guinea pigs. J Nutr. 1992. 122:2019–2029.
Article25. Shanmugasundaram R, Selvaraj RK. Dietary lutein and fish oil interact to alter atherosclerotic lesions in a Japanese quail model of atherosclerosis. J Anim Physiol Anim Nutr (Berl). 2011. 95:762–770.
Article26. Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007. 46:1081–1090.
Article27. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001. 120:1183–1192.
Article28. Nair S, V PC, Arnold C, Diehl AM. Hepatic ATP reserve and efficiency of replenishing: comparison between obese and nonobese normal individuals. Am J Gastroenterol. 2003. 98:466–470.
Article29. McClain CJ, Mokshagundam SP, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I. Mechanisms of non-alcoholic steatohepatitis. Alcohol. 2004. 34:67–79.
Article30. Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990. 9:515–540.
Article31. Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001. 385:20–27.
Article32. Ribaya-Mercado JD, Blumberg JB. Lutein and zeaxanthin and their potential roles in disease prevention. J Am Coll Nutr. 2004. 23:567S–587S.
Article33. Debril MB, Renaud JP, Fajas L, Auwerx J. The pleiotropic functions of peroxisome proliferator-activated receptor gamma. J Mol Med (Berl). 2001. 79:30–47.
Article34. Selvaraj RK, Shanmugasundaram R, Klasing KC. Effects of dietary lutein and PUFA on PPAR and RXR isomer expression in chickens during an inflammatory response. Comp Biochem Physiol A Mol Integr Physiol. 2010. 157:198–203.
Article35. Mai J, Shen X, Shi D, Wei Y, Shen H, Wu M. Effect of lutein on relieving oxidative stress in mice induced by D-galatose. Wei Sheng Yan Jiu. 2010. 39:430–432.36. Ruhl CE, Everhart JE. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology. 2003. 124:1821–1829.
Article37. Preethi KC, Kuttan R. Hepato and reno protective action of Calendula officinalis L. flower extract. Indian J Exp Biol. 2009. 47:163–168.38. Catala A. Lipid peroxidation of membrane phospholipids in the vertebrate retina. Front Biosci (Schol Ed). 2011. 3:52–60.
Article39. Ham WT Jr, Mueller HA, Sliney DH. Retinal sensitivity to damage from short wavelength light. Nature. 1976. 260:153–155.
Article40. Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000. 45:115–134.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet
- Cholesterol-induced inflammation and macrophage accumulation in adipose tissue is reduced by a low carbohydrate diet in guinea pigs
- Pattern of Oral Desensitization in DNCB Presensitized Guinea Pigs
- Effect of coenzyme Q10 and Ardisia japonica Blume on plasma and liver lipids, platelet aggregation, and erythrocyte Na efflux channels in simvastatin-treated guinea pigs
- Lycopene supplementation suppresses oxidative stress induced by a high fat diet in gerbils