Lab Med Online.
2015 Apr;5(2):63-68. 10.3343/lmo.2015.5.2.63.
Performance Evaluation of the Elecsys Neuron-Specific Enolase Assay
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. wlee1@amc.seoul.kr
- KMID: 2312277
- DOI: http://doi.org/10.3343/lmo.2015.5.2.63
Abstract
- BACKGROUND
Neuron-specific enolase (NSE) is an enzyme specifically found in neurons and neuroendocrine tissue. It is a common marker for small cell lung cancer diagnosis and is also useful as a predictor of brain damage. This study evaluates the performance of Elecsys NSE (Roche Diagnostics, Switzerland), an electrochemiluminescent immunoassay.
METHODS
The precision, linearity, limit of detection, and reference interval of the Elecsys NSE, as well as the correlation between Elecsys NSE and ELSA-NSE (Cis-Bio International, France) were evaluated in accordance with the Clinical Laboratory Standards Institute (CLSI) guidelines. PreciControl Tumor Marker (Roche Diagnostics), patient sera, and sera from healthy individuals were used for the analysis.
RESULTS
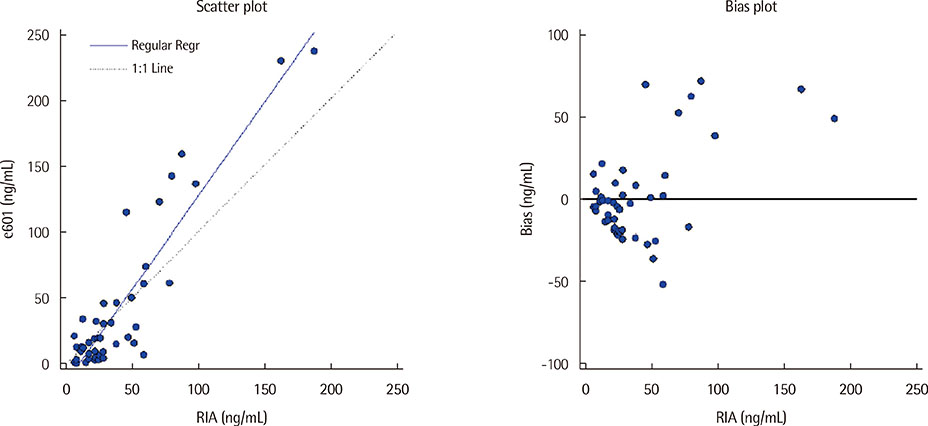
The measured coefficient of variation for the assay was below 3%, and it demonstrated linearity from 0.20 to 234.5 ng/mL. The detection limit was 0.032 ng/mL and the reference interval ranged from 0.05 to 16.3 ng/mL. Compared with the ELSA-NSE assay, the correlation coefficient was 0.9128.
CONCLUSIONS
The Elecsys assay showed suitable precision, linearity, limit of detection and reference range for clinical laboratory use; however, the correlation coefficient of Elecsys NSE as compared to ELSA-NSE was below 0.975. This result may be associated with the use of different monoclonal antibodies in the two different NSE assays. Elecsys NSE demonstrated a high sensitivity without the use of radioactive reagents; therefore, Elecsys NSE will be quite useful for NSE analysis in the clinical laboratory setting.
MeSH Terms
Figure
Reference
-
1. Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci. 2001; 58:902–920.2. Tapia FJ, Polak JM, Barbosa AJ, Bloom SR, Marangos PJ, Dermody C, et al. Neuron-specific enolase is produced by neuroendocrine tumours. Lancet. 1981; 1:808–811.
Article3. Moore BW. Chemistry and biology of two proteins, S-100 and 14-3-2, specific to the nervous system. Int Rev Neurobiol. 1972; 15:215–225.
Article4. Marangos PJ, Polak JM, Pearse AGE. Neuron-specific enolase: a probe for neurons and neuroendocrine cells. Trends Neurosci. 1982; 5:193–196.5. Schmechel D, Marangos PJ, Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature. 1978; 276:834–836.
Article6. Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004; 25:458–511.
Article7. Carney DN, Ihde DC, Cohen MH, Marangos PJ, Bunn PA Jr, Minna JD, et al. Serum neuron-specific enolase: a marker for disease extent and response to therapy of small-cell lung cancer. Lancet. 1982; 1:583–585.
Article8. Ariyoshi Y, Kato K, Ishiguro Y, Ota K, Sato T, Suchi T. Evaluation of serum neuron-specific enolase as a tumor marker for carcinoma of the lung. Gan. 1983; 74:219–225.9. Akoun GM, Scarna HM, Milleron BJ, Bénichou MP, Herman DP. Serum neuron-specific enolase. A marker for disease extent and response to therapy for small-cell lung cancer. Chest. 1985; 87:39–43.10. Molina R, Holdenrieder S, Auge JM, Schalhorn A, Hatz R, Stieber P. Diagnostic relevance of circulating biomarkers in patients with lung cancer. Cancer Biomark. 2010; 6:163–178.
Article11. Ebert W, Hoppe M, Muley T, Drings P. Monitoring of therapy in inoperable lung cancer patients by measurement of CYFRA 21-1, TPA-TP CEA, and NSE. Anticancer Res. 1997; 17:2875–2878.12. Inoue S, Takahashi H, Kaneko K. The fluctuations of neuron-specific enolase (NSE) levels of cerebrospinal fluid during bacterial meningitis: The relationship between the fluctuations of NSE levels and neurological complications or outcome. Acta Paediatr Jpn. 1994; 36:485–488.
Article13. Cunningham RT, Watt M, Winder J, McKinstry S, Lawson JT, Johnston CF, et al. Serum neurone-specific enolase as an indicator of stroke volume. Eur J Clin Invest. 1996; 26:298–303.
Article14. Ondruschka B, Pohlers D, Sommer G, Schober K, Teupser D, Franke H, et al. S100B and NSE as useful postmortem biochemical markers of traumatic brain injury in autopsy cases. J Neurotrauma. 2013; 30:1862–1871.
Article15. Cunningham RT, Johnston CF, Irvine GB, McIlrath EM, McNeill A, Buchanan KD. Development of a radioimmunoassay for neurone specific enolase (NSE) and its application in the study of patients receiving intra hepatic arterial streptozotocin and floxuridine. Clin Chim Acta. 1990; 189:275–286.
Article16. Fu X, Meng M, Zhang Y, Yin Y, Zhang X, Xi R. Chemiluminescence enzyme immunoassay using magnetic nanoparticles for detection of neuron specific enolase in human serum. Anal Chim Acta. 2012; 722:114–118.
Article17. Muley T, Ebert W, Stieber P, Raith H, Holdenrieder S, Nagel D, et al. Technical performance and diagnostic utility of the new Elecsys neuron-specific enolase enzyme immunoassay. Clin Chem Lab Med. 2003; 41:95–103.
Article18. Sterk M, Oenings A, Eymann E, Roos W. Development of a new automated enzyme immunoassay for the determination of neuron-specific enolase. Anticancer Res. 1999; 19:2759–2762.19. Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guideline. CLSI document EP5-A2. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2004.20. Clinical and Laboratory Standards Institute. Evaluation of the Linearity of Quantitative Measurement Procedures: a Statistical Approach; Approved Guideline. CLSI document EP6-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.21. Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guideline. CLSI document EP17-A2. 2nd ed. Clinical and Laboratory Standards Institute;2012.22. Clinical and Laboratory Standards Institute. Defining, Establishing, and verifying reference intervals in the clinical laboratory; approved guideline. CLSI document C28-A3. 3rd ed. Clinical and Laboratory Standards Institute;2008.23. Sturgeon CM, Diamandis EP, editors. Use of Tumor Markers in Clinical Practice: quality Requirements. Washington, DC: American Association for Clinical Chemistry;2009.24. Braga F, Ferraro S, Mozzi R, Dolci A, Panteghini M. Biological variation of neuroendocrine tumor markers chromogranin A and neuron-specific enolase. Clin Biochem. 2013; 46:148–151.
Article25. Mumbarkar PP, Raste AS, Ghadge MS. Significance of tumor markers in lung cancer. Indian J Clin Biochem. 2006; 21:173–176.
Article26. Ku BD. Change of Serum Neuron Specific Enolase Level During Acute Stage of Cerebral Infarction. J Korean Neurol Assoc. 2009; 27:13–18.27. Moritz S, Warnat J, Bele S, Graf BM, Woertgen C. The prognostic value of NSE and S100B from serum and cerebrospinal fluid in patients with spontaneous subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2010; 22:21–31.
Article28. Woo HY, Kim YJ, Park H. Establishment of reference intervals of tumor markers in Korean adults. Korean J Lab Med. 2008; 28:179–184.
Article29. Stern P, Bartos V, Uhrova J, Bezdickova D, Vanickova Z, Tichy V, et al. Performance characteristics of seven neuron-specific enolase assays. Tumour Biol. 2007; 28:84–92.
Article30. Štern P, Bartoš V, Uhrová J, Springer D, Vaníčá Z, Tichý V, et al. The comparability of different neuron-specific enolase immunoassays and its impact on external quality assessment system. Klin Biochem Metab. 2007; 15:21–26.31. Schmitt UM, Stieber P, Hasholzner U, Pahl H, Hofmann K, Fateh-Moghadam A. Methodological and clinical evaluation of two automated enzymatic immunoassays as compared with a radioimmunoassay for neuron-specific enolase. Eur J Clin Chem Clin Biochem. 1996; 34:679–682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of Elecsys Vitamin D Total II Assay Using Roche Modular Analytics E170
- Comparison of the Diagnostic Performance of Elecsys Anti-HCV II and Elecsys and Vitros Anti-HCV Assays
- Increasing the Efficiency of Laboratory Performance by Using the Onboard Dilution Algorithm of the Elecsys Hepatitis B Surface Antigen II Quantitative Assay
- Performance of the Elecsys HIV combi PT Assay Compared to the ARCHITECT HIV Ag/Ab Combo Assay
- Clinical Value of Serum Neuron Specific Enolase in Patients with Undifferentiated Small Cell Carcinoma of the Lung