Lab Anim Res.
2010 Sep;26(3):265-271. 10.5625/lar.2010.26.3.265.
Inhibitory Effects of Korean Red Ginseng Extract on Atopic Dermatitis in NC/Nga Mice
- Affiliations
-
- 1General Medicine & Women's HealthCare, Bayer Korea Ltd, Seoul, Korea.
- 2College of Veterinary Medicine, Kyungpook National University, Daegu, Korea. kskim728@knu.ac.kr
- 3Daegu Bio Industry Center, Daegu, Korea.
- 4Korean Ginseng Center for Most Valuable Products & Ginseng Genetic Resource Bank, Kyung Hee University, Yongin, Korea.
- 5College of Natural Sciences, Keimyung University, Daegu, Korea.
- KMID: 2312076
- DOI: http://doi.org/10.5625/lar.2010.26.3.265
Abstract
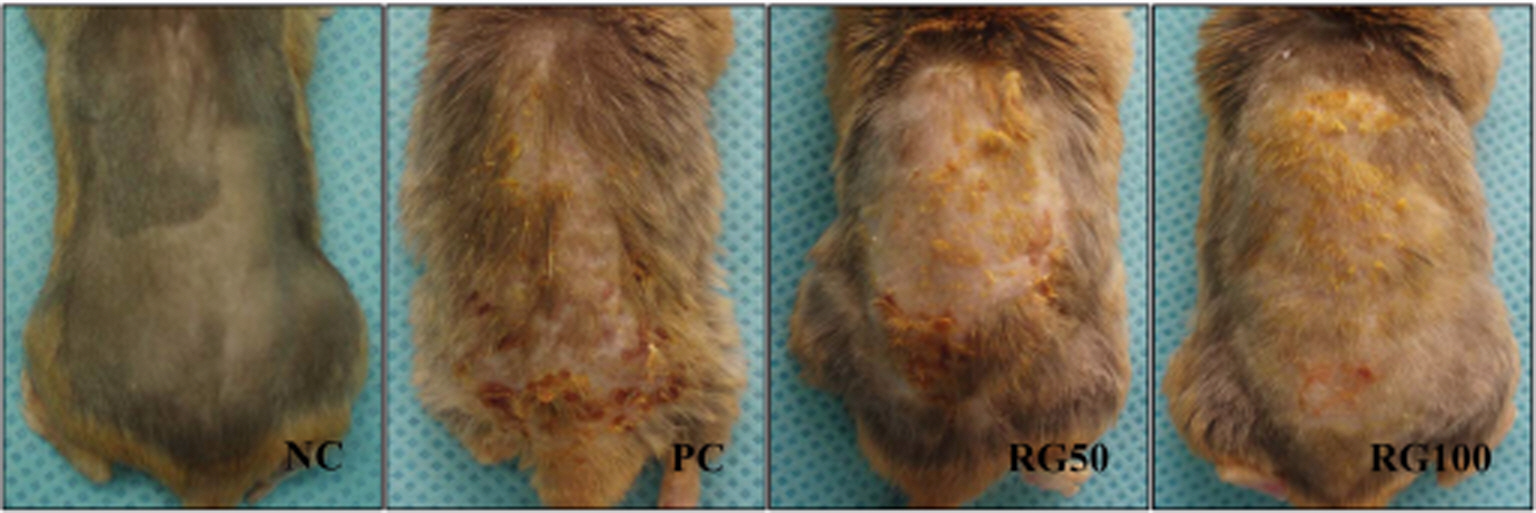
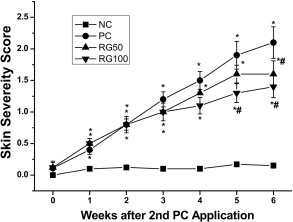
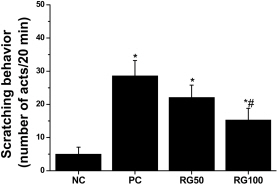
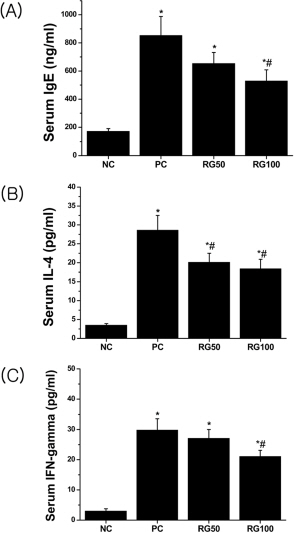
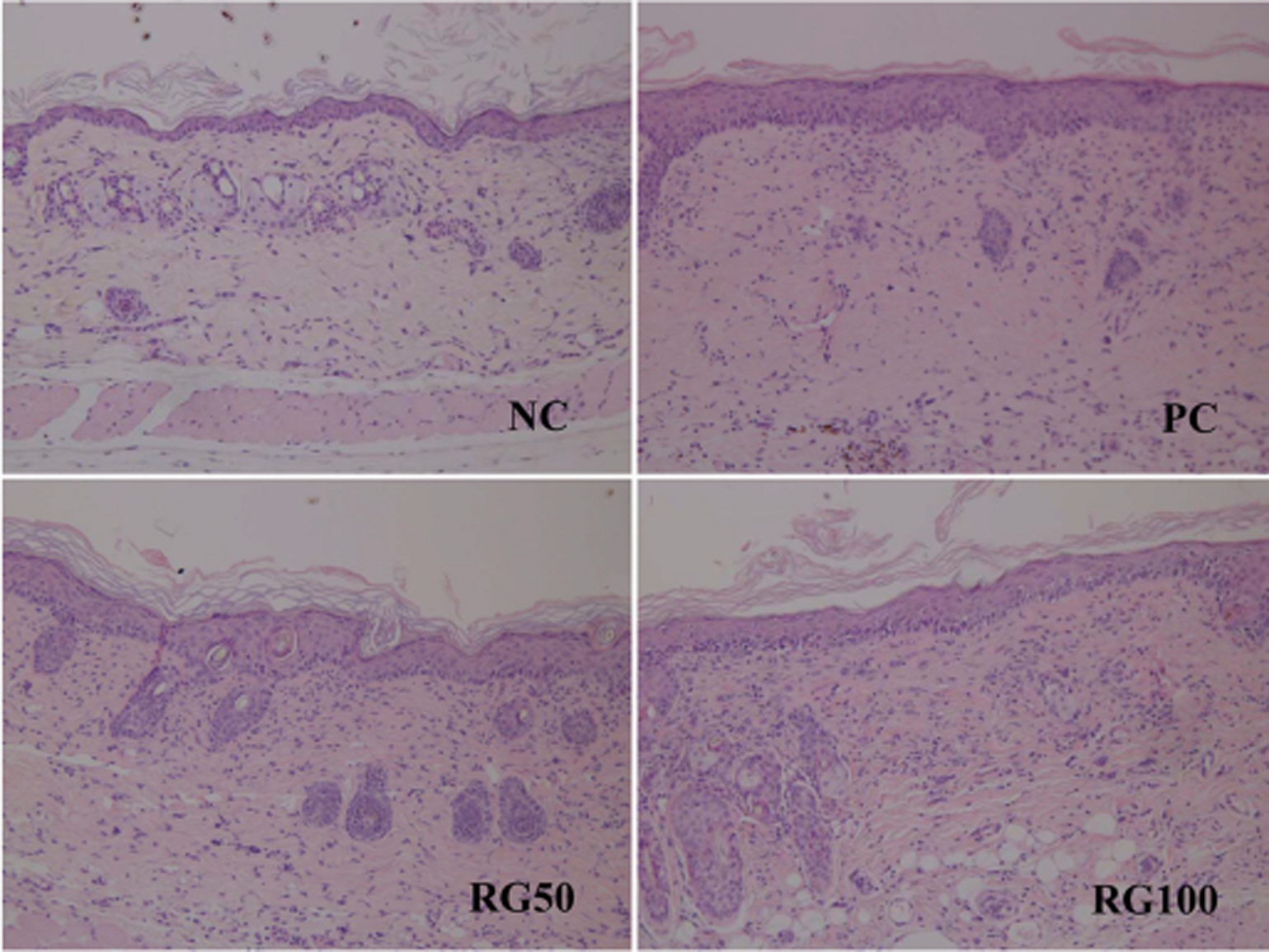
- Atopic dermatitis (AD) is a chronic eczematous skin disease attended by pruritus, erythema, edema, excoriation, and dryness. This study was to evaluate the effects of Korean red ginseng (RG) on AD in NC/Nga mice treated with 1-chloro-2,4,6-trinitrobenzene (picryl chloride; PC). Experimental groups were divided into 4 groups; normal control (NC), PC control, and PC-RG (50 and 100 mg/kg). RG was orally administered every day repeatedly during 6 weeks. The skin lesions in severity score, scratching behavior, serum immunoglobulin E (IgE), interleukin-4 (IL-4) and interferon-gamma (IFN-gamma) levels, and histological appearance were examined. AD-like lesions were developed on the NC/Nga mice by topical PC applications. Oral administration of RG (50 and 100 mg/kg) significantly suppressed the development of AD, as analyzed by a modified SCORAD score. The scratching behavior decreased after RG administration. The levels of serum IgE, IL-4 and IFN-gamma were increased by PC stimulation, but treatment with RG (100 mg/kg) suppressed the increment of the serum IgE, IL-4 and IFN-gamma levels. Histologically, RG inhibited dermatitis lesions such as hypertrophy, hyperkeratosis, and infiltration of inflammatory cells into epidermis and dermis. These results suggest that the administration of RG may be effective in alleviating the AD induced by PC.
MeSH Terms
Figure
Reference
-
Antoine O.M.., Laurent G.M.., Christophe A.C.., Monique M.B.., Felix A.M.., Chantal M.G.., Brice B.C.., Sabine J.P.., Vincent F.., Pierre B.., Daniel V.2002. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma, Am. J. Respir. Crit. Care. 161(6):1790–1796.Attele A.S.., Wu J.A.., Yuan C.S.1999. Ginseng pharmacology: multiple constituents and multiple actions. Biochem. Pharmacol. 58(11):1685–1693.Bae E.A.., Joo H.M.., Shin Y.W.., Kim D.H.2006. Inhibitory Effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol. Pharm. Bull. 29(9):1862–1867.
ArticleChoi S.2002. Epidermis proliferative effect of the Panax ginseng ginsenoside Rb2. Arch. Pharm. Res. 25(1):71–76.Chung S.H.., Choi C.G.., Park S.H.2001. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch. Pharm. Res. 24(3):214–218.
ArticleGrewe M.., Walther S.., Gyufko K.., Czech W.., Schopf E.., Krutmann J.1995. Analysis of the cytokine pattern expressed in situ in inhalant allergen patch test reactions of atopic dermatitis patients. J. Invest. Dermatol. 105(3):407–410.
ArticleGrewe M.., Bruijnzeel-Koomen C.A.., Schopf E.., Thepen T.., Langeveld-Wildschut A.G.., Ruzicka T.., Krutmann J.1998. A role for Th1 and Th2 in the immunopathogenesis of atopic dermatitis. Immunol. Today. 19(8):359–361.Jujo K.., Renz H.., Abe J.., Gelfand E.W.., Leung, D.Y.M. Jujo, K., Renz, H., Abe, J., Gelfand, E.W. and Leung D.Y.M.1992. Decreased interferon gamma and increased interleukin-4 in atopic dermatitis promotes IgE synthesis. J. Allergy Clin. Immunol. 90(3):323–331.Kim S.J.., Kang B.Y.., Cho S.Y.., Sung D.S.., Chang H.K.., Yeom M.H.., Kim D.H.., Sim Y.C.., Lee Y.S.2004. Compound K induces expression of hyaluronan synthetase 2 gene in transformed human keratinocytes and increases hyaluronan in hairless mouse skin. Biochem. Biophys. Res. Commun. 316(2):348–355.Lee E.H.., Cho S.Y.., Kim S.J.., Shin E.S.., Chang H.K.., Lee, Lee, E.H., Cho, S.Y., Kim, S.J., Shin, E.S., Chang, H.K. and Lee T.R.2003. Ginsenoside F1 protects human HaCaT keratinocytes from ultraviolet-B-induced apoptosis by maintaining constant levels of Bcl-2. J. Invest. Dermatol. 121(3):607–613.
ArticleLee J.H.., Lee B.S.., Yang M.S.., Byun B.S.., Kim W.G.., Kim B.H.., Lee S.J.2005. Prevention of photoaging and wrinkle formation in hairless mice dorsal skin by AP3-03. Korean J. Food Sci. Technol. 37:986–996.Lee H.J.., Kim S.R.., Kin J.S.., Moon C.J.., Bae C.S.., Jang J.S.., Jo S.K.., Kim S.H.2006. The effect of red ginseng on ultraviolet B-induced skin damages in mouse. J. Ginseng Res. 30:194–198.Leung D.Y., Bieber T.2003. Atopic dermatitis. Lancet. 361(9352):151–160.
ArticleLeung D.Y.., Boguniewicz M.., Howell M.D.., Nomura I.., Hamid Q.A.2004. New insights into atopic dermatitis. J. Clin. Invest. 113(5):651–657.
ArticleMatsuda H.., Watanabe N.., Geba G.P.., Sperl J.., Tsudzuki M.., Hiroi J.., Matsumoto M.., Ushio H.., Saito S.., Askenase P.W.., Ra C.1997. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 9(3):461–466.
ArticleMatsukura S.., Aihara M.., Hirasawa T.., Ikezawa Z.2005. A effects of TNCB sensitization in DS-Nh mice, serving as a model of atopic dermatitis, in comparison with NC/Nga mice. Int. Arch. Allergy Immunol. 136(2):173–180.Mohrenschlager M.., Darsow U.., Schnopp C.., Ring J.2006. Atopic eczema: what's new? J. Eur. Acad. Dermatol. Venereol. 20(5):503–513.
ArticleMosmann T.R.., Cherwinski H.., Bond M.W.., Giedlin M.A.., Coffman R.L.1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348–2357.Nah S.Y.., Park H.J.., McCleskey E.W.1995. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc. Natl. Acad. Sci. USA. 92(19):8739–8743.Novak N.., Bieber T.2003. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 112(2):252–262.
ArticlePaul W.E.., Seder R.A.1994. Lymphokine responses and cytokines. Cell. 76(2):241–251.Poulsen L.K.., Reimert C.M.., Bindslev-Jensen C.., Hansen M.B.., Bendzen K.1995. Biomolecular regulation of the IgE immune response. In vitro IgE synthesis and spontaneous production of cytokines. Int. Arch. Allergy Immunol. 106(1):55–61.
ArticleRing J.1999. Perspectives of atopic eczema in the third millennium. Curr. Probl. Dermatol. 28:194–204.
ArticleRomagnani S.1994. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 12:227–257.
ArticleSchultz-Larsen F.., Hanifin M.2002. Epidermiologyof atopic dermatitis. Immunol. Allergy Clin. North Am. 22(1):1–24.Sotaniemi E.A.., Haapakoski E.., Rautio A.1995. Ginseng therapy in noninsulin-dependent diabetic patients. Diabetes Care. 18(10):1373–1375.Spergel J.M.., Paller A.S.2003. ).Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 112(6 Suppl):S118–127.
ArticleWatanabe N.., Tomimori Y.., Saito K.., Miura K.., Wada A.., Tsudzuki M.., Fukuda Y.2002. Chymase inhibitor improves dermatitis in NC/Nga mice. Int. Arch. Allergy Immunol. 128(3):229–234.
ArticleYamamoto M.1988. Current status of Ginseng research in Japan. In Proceedings of the International Symposium on Ginseng Research (Ha, H., Yamamoto, M., Joo, C.N. and Kuo, S.C. eds.), pp. 12-26, Life Medical Research Institute, China Medical College, Taichuu.Tomimori Y.., Tanaka Y.., Goto M.., Fukuda Y.2005. Repeated topical challenge with chemical antigen elicits sustained dermatitis in NC/Nga mice in specific-pathogen-free condition. J. Invest. Dermatol. 124(1):119–124.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory and Anti-pruritic Effects of Portulaca oleracea L. Extract Using In Vitro and In Vivo Inflammation Model: LPS-treated Raw264.7 Cells, Keratinocytes, NC/Nga Mice and Hairless SKH-1 Mice
- Therapeutic Effects of Korean Red Ginseng Extract in a Murine Model of Atopic Dermatitis: Anti-pruritic and Anti-inflammatory Mechanism
- Developing an Atopic Dermatitis Model and the Effects of Actinidia Extract on Dermatitis in NC/Nga Mice
- A Study on Tests of Skin Safety and Inhibition of Atopic Dermatitis Using a StoneTouch(R) Infrared Scanner in a Mouse Model
- A Novel Model for Human Atopic Dermatitis: Application of Repeated DNCB Patch in BALB/c Mice, in Comparison with NC/Nga Mice