Anat Cell Biol.
2016 Mar;49(1):21-33. 10.5115/acb.2016.49.1.21.
Modulation of axonal sprouting along rostro-caudal axis of dorsal hippocampus and no neuronal survival in parahippocampal cortices by long-term post-lesion melatonin administration in lithium-pilocarpine model of temporal lobe epilepsy
- Affiliations
-
- 1Department of Physiology, Faculty of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran. ghanjkhani@zums.ac.ir
- 2Department of Anatomical Sciences, Faculty of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran. saeedshus@zums.ac.ir
- KMID: 2308929
- DOI: http://doi.org/10.5115/acb.2016.49.1.21
Abstract
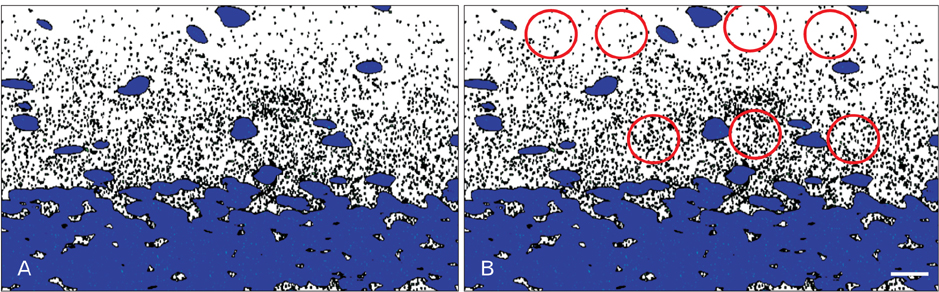
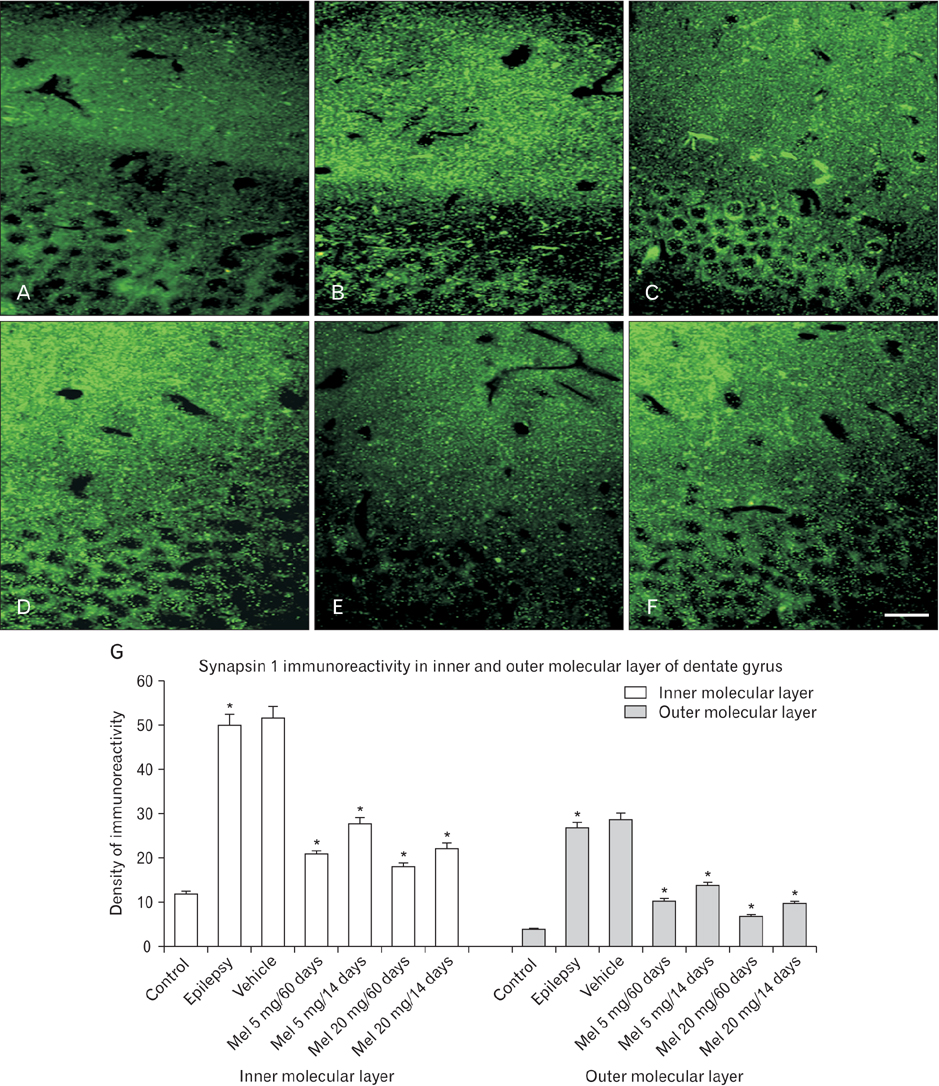
- Feature outcome of hippocampus and extra-hippocampal cortices was evaluated in melatonin treated lithium-pilocarpine epileptic rats during early and chronic phases of temporal lobe epilepsy (TLE). After status epilepticus (SE) induction, 5 and 20 mg/kg melatonin were administered for 14 days or 60 days. All animals were killed 60 days post SE induction and the histological features of the rosrto-caudal axis of the dorsal hippocampus, piriform and entorhinal cortices were evaluated utilizing Nissl, Timm, and synapsin I immunoflorescent staining. Melatonin (20 mg/kg) effect on CA1 and CA3 neurons showed a region-specific pattern along the rostro-caudal axis of the dorsal hippocampus. The number of counted granular cells by melatonin (20 mg/kg) treatment increased along the rostro-caudal axis of the dorsal hippocampus in comparison to the untreated epileptic group. The density of Timm granules in the inner molecular layer of the dentate gyrus decreased significantly in all melatonin treated groups in comparison to the untreated epileptic animals. The increased density of synapsin I immunoreactivity in the outer molecular layer of the dentate gyrus of untreated epileptic rats showed a profound decrease following melatonin treatment. There was no neuronal protection in the piriform and entorhinal cortices whatever the melatonin treatment. Long-term melatonin administration as a co-adjuvant probably could reduce the post-lesion histological consequences of TLE in a region-specific pattern along the rostro-caudal axis of the dorsal hippocampus.
MeSH Terms
Figure
Cited by 1 articles
-
Effect of cypermethrin on the postnatal development of the medulla oblongata and the possible protective role of melatonin in albino rats
Marwa A. Al-Gholam, Noha M. Issa
Anat Cell Biol. 2020;53(4):460-470. doi: 10.5115/acb.20.193.
Reference
-
1. Jung KH, Chu K, Lee ST, Kim JH, Kang KM, Song EC, Kim SJ, Park HK, Kim M, Lee SK, Roh JK. Region-specific plasticity in the epileptic rat brain: a hippocampal and extrahippocampal analysis. Epilepsia. 2009; 50:537–549.2. Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J Jr. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005; 46:470–472.3. Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011; 365:919–926.4. Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008; 7:514–524.5. Maestroni GJ, Conti A, Pierpaoli W. Role of the pineal gland in immunity: II. Melatonin enhances the antibody response via an opiatergic mechanism. Clin Exp Immunol. 1987; 68:384–391.6. Jou MJ, Peng TI, Reiter RJ, Jou SB, Wu HY, Wen ST. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J Pineal Res. 2004; 37:55–70.7. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004; 36:1–9.8. Imbesi M, Uz T, Manev H. Role of melatonin receptors in the effects of melatonin on BDNF and neuroprotection in mouse cerebellar neurons. J Neural Transm (Vienna). 2008; 115:1495–1499.9. Rennie K, De Butte M, Pappas BA. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J Pineal Res. 2009; 47:313–317.10. Moriya T, Horie N, Mitome M, Shinohara K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J Pineal Res. 2007; 42:411–418.11. Ramirez-Rodriguez G, Ortíz-López L, Domínguez-Alonso A, Benítez-King GA, Kempermann G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res. 2011; 50:29–37.12. Benítez-King G. Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. J Pineal Res. 2006; 40:1–9.13. Costa-Lotufo LV, Fonteles MM, Lima IS, de Oliveira AA, Nascimento VS, de Bruin VM, Viana GS. Attenuating effects of melatonin on pilocarpine-induced seizures in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2002; 131:521–529.14. Lima E, Cabral FR, Cavalheiro EA, Naffah-Mazzacoratti Mda G, Amado D. Melatonin administration after pilocarpine-induced status epilepticus: a new way to prevent or attenuate postlesion epilepsy? Epilepsy Behav. 2011; 20:607–612.15. Chung SY, Han SH. Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J Pineal Res. 2003; 34:95–102.16. De Lima E, Soares JM Jr, del Carmen Sanabria Garrido Y, Gomes Valente S, Priel MR, Chada Baracat E, Abrao Cavalheiro E, da Graça Naffah-Mazzacoratti M, Amado D. Effects of pinealectomy and the treatment with melatonin on the temporal lobe epilepsy in rats. Brain Res. 2005; 1043:24–31.17. Lee SH, Chun W, Kong PJ, Han JA, Cho BP, Kwon OY, Lee HJ, Kim SS. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J Pineal Res. 2006; 40:79–85.18. Ravizza T, Gagliardi B, Noe F, Boér K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008; 29:142–160.19. Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008; 172:143–157.20. Epsztein J, Represa A, Jorquera I, Ben-Ari Y, Cépel V. Recurrent mossy fibers establish aberrant kainate receptor-operated synapses on granule cells from epileptic rats. J Neurosci. 2005; 25:8229–8239.21. Rigoulot MA, Koning E, Ferrandon A, Nehlig A. Neuroprotective properties of topiramate in the lithium-pilocarpine model of epilepsy. J Pharmacol Exp Ther. 2004; 308:787–795.22. Frotscher M, Jonas P, Sloviter RS. Synapses formed by normal and abnormal hippocampal mossy fibers. Cell Tissue Res. 2006; 326:361–367.23. Goffin K, Nissinen J, Van Laere K, Pitkänen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol. 2007; 205:501–505.24. Jung S, Jones TD, Lugo JN Jr, Sheerin AH, Miller JW, D'Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007; 27:13012–13021.25. Danzer SC, He X, Loepke AW, McNamara JO. Structural plasticity of dentate granule cell mossy fibers during the development of limbic epilepsy. Hippocampus. 2010; 20:113–124.26. Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res. 2007; 163:601–613.27. Stoop R, Pralong E. Functional connections and epileptic spread between hippocampus, entorhinal cortex and amygdala in a modified horizontal slice preparation of the rat brain. Eur J Neurosci. 2000; 12:3651–3663.28. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972; 32:281–294.29. Zheng Y, Moussally J, Cash SS, Karnam HB, Cole AJ. Intravenous levetiracetam in the rat pilocarpine-induced status epilepticus model: behavioral, physiological and histological studies. Neuropharmacology. 2010; 58:793–798.30. Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997; 17:3727–3738.31. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Syndney: Academic Press;1982.32. Holmes GL, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. Mossy fiber sprouting after recurrent seizures during early development in rats. J Comp Neurol. 1999; 404:537–553.33. Van der Zee CE, Rashid K, Le K, Moore KA, Stanisz J, Diamond J, Racine RJ, Fahnestock M. Intraventricular administration of antibodies to nerve growth factor retards kindling and blocks mossy fiber sprouting in adult rats. J Neurosci. 1995; 15(7 Pt 2):5316–5323.34. Peredery O, Persinger MA, Parker G, Mastrosov L. Temporal changes in neuronal dropout following inductions of lithium/pilocarpine seizures in the rat. Brain Res. 2000; 881:9–17.35. Poirier JL, Capek R, De Koninck Y. Differential progression of Dark Neuron and Fluoro-Jade labelling in the rat hippocampus following pilocarpine-induced status epilepticus. Neuroscience. 2000; 97:59–68.36. Tanti A, Belzung C. Neurogenesis along the septo-temporal axis of the hippocampus: are depression and the action of antidepressants region-specific? Neuroscience. 2013; 252:234–252.37. Bragdon AC, Taylor DM, Wilson WA. Potassium-induced epileptiform activity in area CA3 varies markedly along the septotemporal axis of the rat hippocampus. Brain Res. 1986; 378:169–173.38. Ashton D, Van Reempts J, Haseldonckx M, Willems R. Dorsalventral gradient in vulnerability of CA1 hippocampus to ischemia: a combined histological and electrophysiological study. Brain Res. 1989; 487:368–372.39. Hassan AH, von Rosenstiel P, Patchev VK, Holsboer F, Almeida OF. Exacerbation of apoptosis in the dentate gyrus of the aged rat by dexamethasone and the protective role of corticosterone. Exp Neurol. 1996; 140:43–52.40. Banasr M, Soumier A, Hery M, Mocaër E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006; 59:1087–1096.41. Soumier A, Banasr M, Lortet S, Masmejean F, Bernard N, Kerkerian-Le-Goff L, Gabriel C, Millan MJ, Mocaer E, Daszuta A. Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology. 2009; 34:2390–2403.42. Paizanis E, Renoir T, Lelievre V, Saurini F, Melfort M, Gabriel C, Barden N, Mocaër E, Hamon M, Lanfumey L. Behavioural and neuroplastic effects of the new-generation antidepressant agomelatine compared to fluoxetine in glucocorticoid receptorimpaired mice. Int J Neuropsychopharmacol. 2010; 13:759–774.43. Morley-Fletcher S, Mairesse J, Soumier A, Banasr M, Fagioli F, Gabriel C, Mocaer E, Daszuta A, McEwen B, Nicoletti F, Maccari S. Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats. Psychopharmacology (Berl). 2011; 217:301–313.44. Rainer Q, Xia L, Guilloux JP, Gabriel C, Mocaër E, Hen R, Enhamre E, Gardier AM, David DJ. Beneficial behavioural and neurogenic effects of agomelatine in a model of depression/anxiety. Int J Neuropsychopharmacol. 2012; 15:321–335.45. Ramírez-Rodríguez G, Klempin F, Babu H, Benítez-King G, Kempermann G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology. 2009; 34:2180–2191.46. Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006; 59:81–91.47. Lynch M, Sutula T. Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol. 2000; 83:693–704.48. Siddiqui AH, Joseph SA. CA3 axonal sprouting in kainateinduced chronic epilepsy. Brain Res. 2005; 1066:129–146.49. Dagyte G, Luiten PG, De Jager T, Gabriel C, Mocaër E, Den Boer JA, Van der Zee EA. Chronic stress and antidepressant agomelatine induce region-specific changes in synapsin I expression in the rat brain. J Neurosci Res. 2011; 89:1646–1657.50. Thind KK, Yamawaki R, Phanwar I, Zhang G, Wen X, Buckmaster PS. Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy. J Comp Neurol. 2010; 518:647–667.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mossy fiber Synaptic Reorganization and the Pattern of Sprouting in the Pilocarpine Epilepsy Model
- Morphological Alterations of Hippocampus in Temporal Lobe Epilepsy: Cell Loss, Synaptic Reorganization, Cell Birth
- Effect of MK-801 on Neuronal Cell Loss and Fos Expression of Hippocampus in Lithium-Pilocarpine Induced Status Epilepticus
- Antiepileptic and Neuroprotective Effect of Ketamine in Lithium-Pilocarpine Induced Status Epilepticus Rat Model
- Effect of calcium channel blockers on the hippocampus of Lithium-Pilocarpine induced status epilepticus rat model