Korean Diabetes J.
2009 Dec;33(6):466-474. 10.4093/kdj.2009.33.6.466.
Treatment of Type 1 Diabetes through Genetically Engineered K-cell Transplantation in a Mouse Model
- Affiliations
-
- 1Department of Internal Medicine, Incheon St. Mary's Hospital, The Catholic University of Korea, Incheon, Korea. sungdaem@gmail.com
- 2Research Institute of Immunobiology, The Catholic University of Korea, Seoul, Korea.
- KMID: 2298052
- DOI: http://doi.org/10.4093/kdj.2009.33.6.466
Abstract
- BACKGROUND
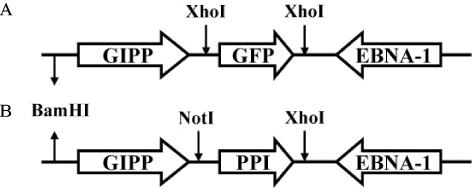
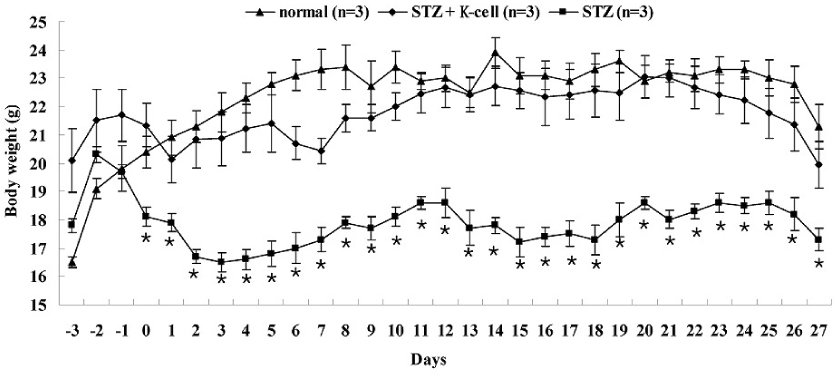
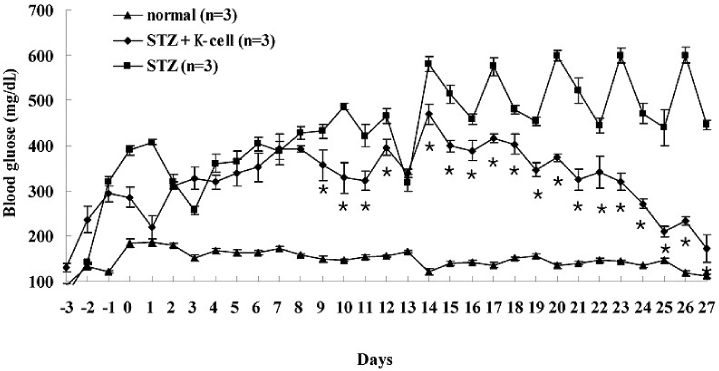
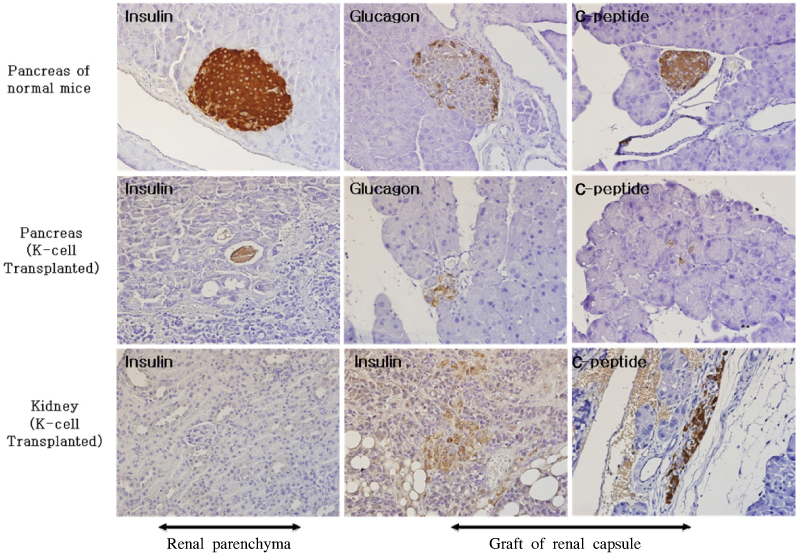
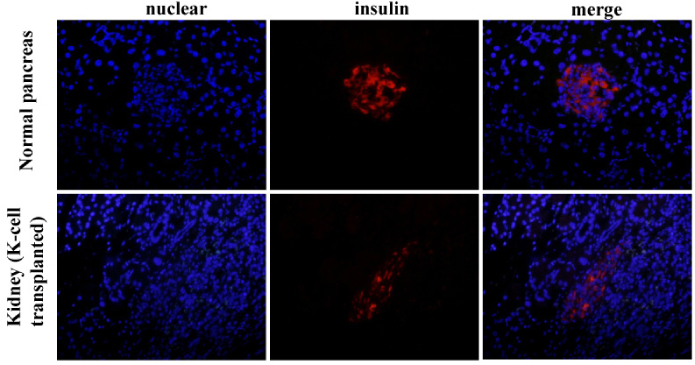
K-cells function as targets for insulin gene therapy. In a previous study, we constructed EBV-based plasmids expressing rat preproinsulin controlled by glucose-dependent insulinotropic polypeptide promoters. In the present study, we attempted to correct hyperglycemia in vivo using genetically engineered K-cells in a mouse model of type 1 diabetes. METHODS: K-cells expressing insulin were transplanted under the kidney capsules of STZ-induced diabetic mice. The blood glucose levels and body weights of the experimental animals were measured daily. After four weeks, the mice were injected intra-peritoneally with 2 g/kg glucose following a 6 hr fast. Blood glucose levels were measured immediately following glucose injections. All animals were sacrificed at the end of the glucose tolerance study, and pancreas and graft-bearing kidney tissue samples were stained with antibodies against insulin, glucagon, and C-peptide. RESULTS: The body weights of K-cell-transplanted diabetic mice increased after transplantation, whereas those of untreated diabetic control mice continued to decline. The blood glucose levels of K-cell-transplanted diabetic mice decreased gradually during the two weeks following transplantation. After intra-peritoneal injection of glucose into K-cell-transplanted diabetic mice, blood glucose levels increased at 30 minutes, and were restored to the normal range between 60 and 90 minutes, while untreated control diabetic mice continued to experience hyperglycemia. Kidney capsules containing transplanted K-cells were removed, and sections were stained with anti-insulin antibodies. We detected insulin-positive cells in the kidney capsules of K-cell-transplanted diabetic mice, but not in untreated control mice. CONCLUSION: We detected glucose-dependent insulin secretion in genetically engineered K-cells in a mouse model of type 1 diabetes. Our results suggest that genetically modified insulin producing K-cells may act as surrogate beta-cells to effectively treat type 1 diabetes.
MeSH Terms
-
Animals
Antibodies
Blood Glucose
Body Weight
C-Peptide
Capsules
Gastric Inhibitory Polypeptide
Genetic Therapy
Glucagon
Glucose
Herpesvirus 4, Human
Hyperglycemia
Insulin
Kidney
Mice
Pancreas
Plasmids
Protein Precursors
Rats
Reference Values
Transplants
Antibodies
Blood Glucose
C-Peptide
Capsules
Gastric Inhibitory Polypeptide
Glucagon
Glucose
Insulin
Protein Precursors
Figure
Reference
-
1. Levine F, Leibowitz G. Towards gene therapy of diabetes mellitus. Mol Med Today. 1999. 5:165–171.2. Morral N. Gene therapy for type 1 diabetes. New approaches. Minerva Med. 2004. 95:93–104.3. Yoon JW, Jun HS. Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med. 2002. 8:62–68.4. Steiner DF, Rouille Y, Gong Q, Martin S, Carroll R, Chan SJ. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes Metab. 1996. 22:94–104.5. Tang SC, Sambanis A. Development of genetically engineered human intestinal cells for regulated insulin secretion using rAAV-mediated gene transfer. Biochem Biophys Res Commun. 2003. 303:645–652.6. Nett PC, Sollinger HW, Alam T. Hepatic insulin gene therapy in insulin-dependent diabetes mellitus. Am J Transplant. 2003. 3:1197–1203.7. Stewart C, Taylor NA, Green IC, Docherty K, Bailey CJ. Insulin-releasing pituitary cells as a model for somatic cell gene therapy in diabetes mellitus. J Endocrinol. 1994. 142:339–343.8. Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe MM, Kieffer TJ. Glucose-dependent insulin release from genetically engineered K cells. Science. 2000. 290:1959–1962.9. Ramshur EB, Rull TR, Wice BM. Novel insulin/GIP co-producing cell lines provide unexpected insights into Gut K-cell function in vivo. J Cell Physiol. 2002. 192:339–350.10. Corbett JA. K cells: a novel target for insulin gene therapy for the prevention of diabetes. Trends Endocrinol Metab. 2001. 12:140–142.11. Min KA, Oh ST, Yoon KH, Kim CK, Lee SK. Prolonged gene expression in primary porcine pancreatic cells using an Epstein-Barr virus-based episomal vector. Biochem Biophys Res Commun. 2003. 305:108–115.12. Son JK, Oh ST, Cho SK, Yoon KH, Lee SK. Mechanism of prolonged gene expression by Epstein-Barr virus-based plasmid in porcine cells. Xenotransplantation. 2006. 13:560–565.13. Mizuguchi H, Hosono T, Hayakawa T. Long-term replication of Epstein-Barr virus-derived episomal vectors in the rodent cells. FEBS Lett. 2000. 472:173–178.14. Kim JH, Moon SD, Ko SH, Ahn YB, Song KH, Lim HS, Lee SK, Yoo SJ, Son HS, Yoon KH, Cha BY, Son HY, Kim SJ, Han JH. Glucose-dependent Insulin secretion from genetically engineered K-cells using EBV-based episomal vector. J Korean Diabetes Assoc. 2007. 31:9–21.15. Takeshita F, Kodama M, Yamamoto H, Ikarashi Y, Ueda S, Teratani T, Yamamoto Y, Tamatani T, Kanegasaki S, Ochiya T, Quinn G. Streptozotocin-induced partial beta cell depletion in nude mice without hyperglycaemia induces pancreatic morphogenesis in transplanted embryonic stem cells. Diabetologia. 2006. 49:2948–2958.16. Zhang Y, Yao L, Shen K, Xu M, Zhou P, Yang W, Liu X, Qin X. Genetically engineered K cells provide sufficient insulin to correct hyperglycemia in a nude murine model. Acta Biochim Biophys Sin (Shanghai). 2008. 40:149–157.17. Han J, Lee HH, Kwon H, Shin S, Yoon JW, Jun HS. Engineered enteroendocrine cells secrete insulin in response to glucose and reverse hyperglycemia in diabetic mice. Mol Ther. 2007. 15:1195–1202.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cryopreservation of mouse resources
- Insights into granulosa cell tumors using spontaneous or genetically engineered mouse models
- Mouse models of breast cancer in preclinical research
- Genetically Engineered Mouse Models for Drug Development and Preclinical Trials
- Application of engineered cell sheets composed of human islets and supporting stem cells enhances the outcome of islet cell transplantation in vitro and in vivo