Korean J Urol.
2006 Jul;47(7):779-785. 10.4111/kju.2006.47.7.779.
Location of Brain Electrical Source Activation according to Visually Stimulated Sexual Arousal: A Cross Spectral Analysis using Low Resolution Brain Electromagnetic Tomography (LORETA)
- Affiliations
-
- 1Department of Urology, School of Medicine, Gyeongsang National University, Jinju, Korea. hyunjs@gshp.gsnu.ac.kr
- 2Department of Neurology, School of Medicine, Gyeongsang National University, Jinju, Korea.
- KMID: 2294322
- DOI: http://doi.org/10.4111/kju.2006.47.7.779
Abstract
-
PURPOSE: Low resolution brain electromagnetic tomography (LORETA) is a kind of functional imaging technique and it is also an up-to-date technique for conducting electroencephalography (EEG) analysis. We tried to investigate the locations on the cerebral cortex that are activated by visually stimulated sexual arousal.
MATERIALS AND METHODS
Thirty-three male volunteers (age range: 24.7+/-1.7 years) among all the right-handed medical students at our university were enrolled in this study. The EEGs included the segments recorded during resting, watching a music-video, intermission and watching a porno-video. The LORETA images of the cross-spectral analysis were obtained with using segments of LORETA-KEY (KEY Institute for Brain-Mind Research, Switzerland) software.
RESULTS
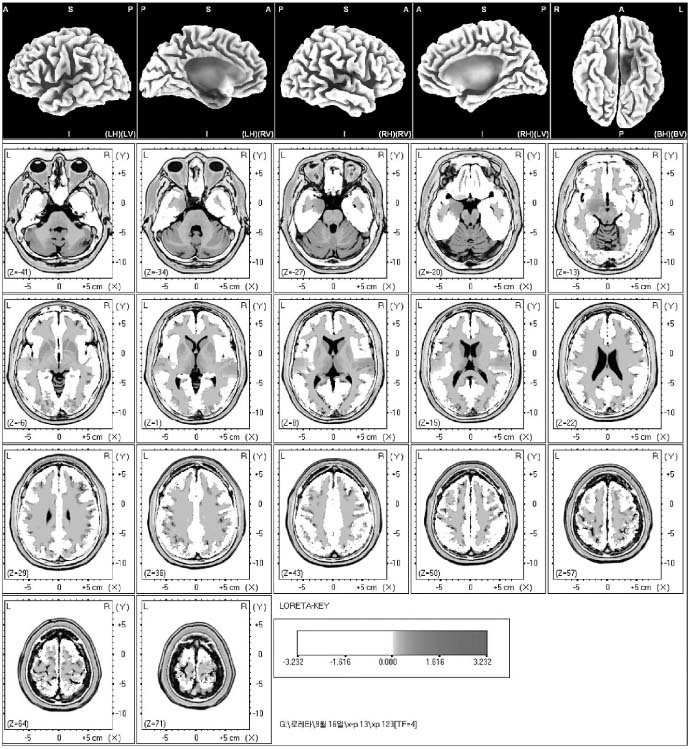
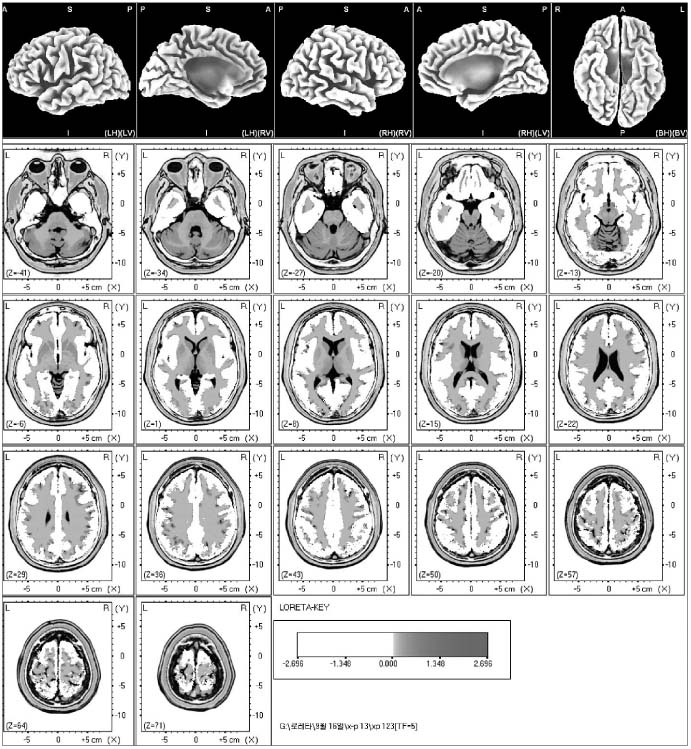
In the statistical nonparametric maps (SnPM) of each spectrum and the delta, theta and alpha waves did not show the increased current density. The beta 1, 2 and 3 activity showed the point of maximal current densities in the anterior parahippocampal gyrus of the left limbic lobe and the superior temporal gyrus of both temporal lobes, the superior temporal gyrus of the right temporal lobe, the precuneus of the right parietal lobe, the medial frontal gyrus of the left frontal lobe, the middle occipital gyrus of the right occipital lobe, the superior temporal gyrus of both temporal lobes and the superior frontal gyrus of the right frontal lobe.
CONCLUSIONS
The sexual arousal by visual stimulation may activate the anterior parahippocampal gyrus of the left limbic lobe, the superior temporal gyrus of both temporal lobes, the precuneus of the right parietal lobe, the medial frontal gyrus of the left frontal gyrus, and the middle occipital gyrus of the right occipital lobe.
Keyword
MeSH Terms
Figure
Reference
-
1. Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994. 18:49–65.2. Kim MR, Kim KR, Ha CK, Choi SH, Lee IK. Comparative study between visual analysis and low resolution electromagnetic tomography (LORETA) method in the localizaion of epileptiform discharges. J Korean Neurol Assoc. 2002. 20:164–168.3. Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 1988. New York: Thieme;1.4. Towle VL, Bolanos J, Suarez D, Tan K, Grzeszczuk R, Levin DN, et al. The spatial location of EEG electrodes: locating the best-fitting sphere relative to cortical anatomy. Electroencephalogr Clin Neurophysiol. 1993. 86:1–6.5. Stoleru S, Gregoire MC, Gerard D, Decety J, Lafarge E, Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999. 28:1–21.6. Bocher M, Chisin R, Parag Y, Freedman N, Meir Weil Y, Lester H, et al. Cerebral activation associated with sexual arousal in response to a pornographic clip: A 15O-H2O PET study in heterosexual men. Neuroimage. 2001. 14:105–117.7. Redoute J, Stoleru S, Gregoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000. 11:162–177.8. Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996. 112:103–111.9. Phelps ME, Mazziotta JC. Positron emission tomography: human brain function and biochemistry. Science. 1985. 228:799–809.10. Stehling MK, Turner R, Mansfield P. Echo-planar imaging: magnetic resonance imaging in a fraction of a second. Science. 1991. 254:43–50.11. Longworth C, Honey G, Sharma T. Science, medicine, and the future: functional magnetic resonance imaging in neuropsychiatry. BMJ. 1999. 319:1551–1554.12. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990. 87:9868–9872.13. Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002. 125:1014–1023.14. Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001. 21:RC165.15. Karama S, Lecours AR, Leroux JM, Bourgouim P, Beaudoin G, Joubert S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2005. 16:1–13.16. Park K, Seo JJ, Kang HK, Ryu SB, Kim HJ, Jeong GW. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res. 2001. 13:73–81.17. Park KS, Kang HK, Seo JJ, Kim HJ, Ryu SB, Jeong GW. Blood-oxygenation-level-dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology. 2001. 57:1189–1194.18. Stehling MK, Turner R, Mansfield P. Echo-planar imaging: magnetic resonance imaging in a fraction of a second. Science. 1991. 254:43–50.19. Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990. 14:68–78.20. Mouras H, Stoleru S, Bittoun J, Glutron D, Pelegrini-Issac M, Paradis AL, et al. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. Neuroimage. 2003. 20:855–869.21. Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Moller HJ, et al. Integration of fMRI and simultaneous EEG : towards a comprehensive understanding of localization and time-corse of brain activity in target detection. Neuroimage. 2004. 22:83–94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Location of Brain Electrical Source Activation by Visually Stimulated Sexual Arousal in Young Men and Women: a Cross Spectral Analysis using Low Resolution Brain Electromagnetic Tomography (LORETA)
- Low-Resolution Electromagnetic Tomography (LORETA) Source Imaging Compared with Structural Brain Imaging in Patients having Organic Brain Lesion
- Comparative Study between Visusal Analysis and Low Resolution Electromagnetic Tomography (LORETA) Method in the Localization of Epileptiform Discharges
- Comparison of Low Resolution Electromagnetic Tomography Imaging Between Subjects With Mild and Severe Obstructive Sleep Apnea Syndrome: A Preliminary Study
- Dipole Source Localization and Low Resolution Electromagnetic Tomography(LORETA) of Interictal Spikes in Mesial and Lateral Temporal Lobe Epilepsy