J Breast Cancer.
2012 Dec;15(4):381-387. 10.4048/jbc.2012.15.4.381.
HER2 Status by Standardized Immunohistochemistry and Silver-Enhanced In Situ Hybridization in Korean Breast Cancer
- Affiliations
-
- 1Department of Pathology, Yeungnam University College of Medicine, Daegu, Korea.
- 2Department of Pathology, University of Ulsan College of Medicine, Seoul, Korea. gygong@amc.seoul.kr
- 3Department of Pathology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 4Department of Pathology, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 5Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Pathology, Chonnam National University School of Medicine, Gwangju, Korea.
- 7Department of Pathology, Chungnam National University College of Medicine, Daejeon, Korea.
- 8Department of Pathology, Soonchunhyang University Hospital, Seoul, Korea.
- 9Department of Pathology, Yonsei University Severance Hospital, Seoul, Korea.
- KMID: 2286433
- DOI: http://doi.org/10.4048/jbc.2012.15.4.381
Abstract
- PURPOSE
Amplification of the human epidermal growth factor receptor 2 (HER2) gene occurs in 18% to 20% of breast cancers, and it is recognized as a prognostic and predictive marker. We investigated the HER2 status in Korean breast cancer by immunohistochemistry (IHC) and silver-enhanced in situ hybridization (SISH), as the first step toward building a nationwide quality assurance program for HER2 testing.
METHODS
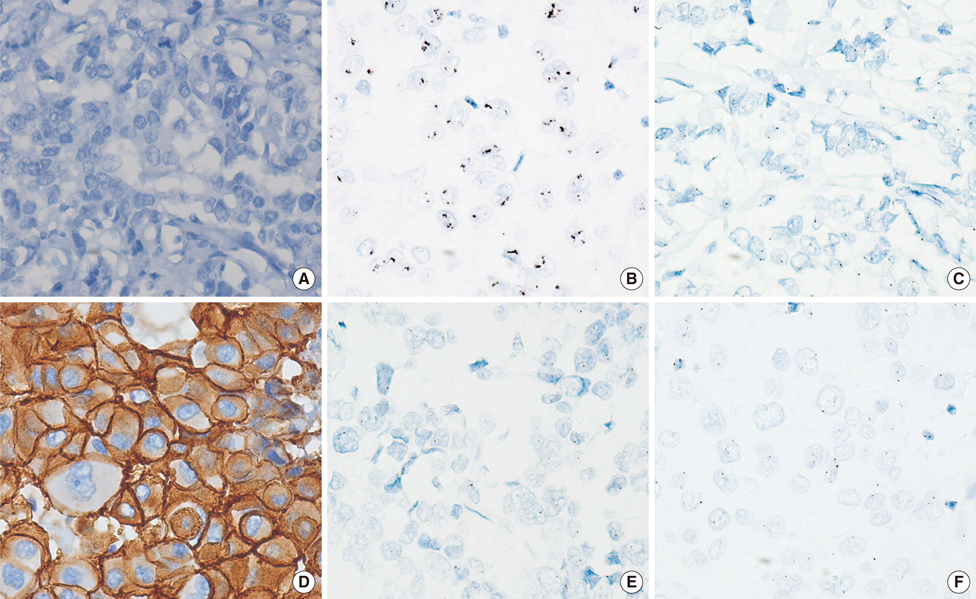
A total of 1,198 breast carcinoma samples were collected from six institutions and IHC and SISH were performed using tissue microarrays in central laboratories. The results were compared to those of local laboratories.
RESULTS
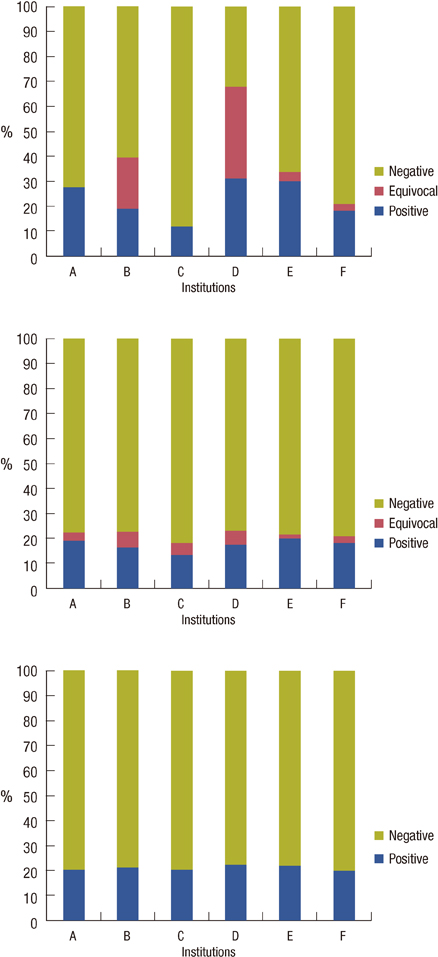
Available data were obtained from 959 samples. Central IHC results were negative, equivocal, and positive for 756 (78.8%; range among institutions, 76.8-81.8%), 37 (3.9%; 1.9-6.2%), and 166 (17.3%; 13.6-20%), respectively. SISH results were negative, equivocal, and positive for 756 (78.8%; 77.4-79.9%), 2 (0.2%; 0-0.7%), and 201 (21%; 20.1-22.2%), respectively. HER2 gene amplification was observed in 4.4%, 19%, and 73.9% of the negative, equivocal and positive groups stratified by local IHC results, respectively. When central SISH was considered to be the gold standard method for measuring HER2 status, the false-negative and false-positive rates of local IHC were 14.4% (29/201) and 7.1% (54/756). The concordance rate between central IHC and SISH was 98.4%.
CONCLUSION
Central IHC and SISH markedly decreased the interlaboratory variability of HER2 status and the results of the two were highly concordant. The quality control program for HER2 testing must be focused on decreasing both the false negativity and positivity of IHC in local laboratories.
MeSH Terms
Figure
Cited by 1 articles
-
Positive Expression of Insulin-Like Growth Factor-1 Receptor Is Associated with a Positive Hormone Receptor Status and a Favorable Prognosis in Breast Cancer
Su-Jin Shin, Gyungyub Gong, Hee Jin Lee, Jun Kang, Young Kyung Bae, Ahwon Lee, Eun Yoon Cho, Ji Shin Lee, Kwang-Sun Suh, Dong Wha Lee, Woo Hee Jung
J Breast Cancer. 2014;17(2):113-120. doi: 10.4048/jbc.2014.17.2.113.
Reference
-
1. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.
Article2. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001. 344:783–792.
Article3. Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009. 14:320–368.
Article4. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007. 25:5287–5312.
Article5. Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009. 27:1323–1333.
Article6. Dietel M, Ellis IO, Höfler H, Kreipe H, Moch H, Dankof A, et al. Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007. 451:19–25.
Article7. Kang J, Kwon GY, Lee YH, Gong G. Comparison of silver-enhanced in situ hybridization and fluorescence in situ hybridization for HER2 gene status in breast carcinomas. J Breast Cancer. 2009. 12:235–240.
Article8. Sung WJ, Park SJ, Gu MJ, Bae YK. Automated silver-enhanced in situ hybridization for evaluation of HER2 gene status in breast carcinoma: comparison with fluorescence in situ hybridization and immunohistochemistry. Korean J Pathol. 2010. 44:28–34.
Article9. Paik S, Bryant J, Tan-Chiu E, Romond E, Hiller W, Park K, et al. Real-world performance of HER2 testing: National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002. 94:852–854.
Article10. Roche PC, Suman VJ, Jenkins RB, Davidson NE, Martino S, Kaufman PA, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst. 2002. 94:855–857.
Article11. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.
Article12. van der Vegt B, de Bock GH, Bart J, Zwartjes NG, Wesseling J. Validation of the 4B5 rabbit monoclonal antibody in determining Her2/neu status in breast cancer. Mod Pathol. 2009. 22:879–886.
Article13. De P, Smith BR, Leyland-Jones B. Human epidermal growth factor receptor 2 testing: where are we? J Clin Oncol. 2010. 28:4289–4292.
Article14. Park K, Han S, Kim JY, Kim HJ, Kwon JE, Gwak G. Silver-enhanced in situ hybridization as an alternative to fluorescence in situ hybridization for assaying HER2 amplification in clinical breast cancer. J Breast Cancer. 2011. 14:276–282.
Article15. Park S, Park HS, Koo JS, Yang WI, Kim SI, Park BW. Breast cancers presenting luminal B subtype features show higher discordant human epidermal growth factor receptor 2 results between immunohistochemistry and fluorescence in situ hybridization. Cancer. 2012. 118:914–923.
Article16. Bilous M, Farshid G. Assessment of human epidermal growth factor receptor 2 (HER2) amplification by in situ hybridization (ISH): findings from a nationwide program in Australia. 32nd Annual CTRC-AACR San Antonio Breast Cancer Symposium. 2009. Abstract #5100.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Automated Silver-enhanced In Situ Hybridization for Evaluation of HER2 Gene Status in Breast Carcinoma: Comparison with Fluorescence In Situ Hybridization and Immunohistochemistry
- Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer
- HER2 Status in Gastric Adenocarcinomas Assessed by Immunohistochemistry, Automated Silver-Enhanced In Situ Hybridization and Fluorescence In Situ Hybridization
- The Comparison of Automated Silver in situ Hybridization and Fluorescence in situ Hybridization for Evaluating HER2 Gene Amplification in Breast Carcinoma
- Comparison of Silver-Enhanced in situ Hybridization and Fluorescence in situ Hybridization for HER2 Gene Status in Breast Carcinomas