Comparison of Silver-Enhanced in situ Hybridization and Fluorescence in situ Hybridization for HER2 Gene Status in Breast Carcinomas
- Affiliations
-
- 1Department of Pathology, Military Manpower Administration, Government of Republic of Korea, Seoul, Korea.
- 2Department of Pathology, Chung-Ang University of Medical Center, Seoul, Korea.
- 3Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. gygong@amc.seoul.kr
- KMID: 2175512
- DOI: http://doi.org/10.4048/jbc.2009.12.4.235
Abstract
- PURPOSE
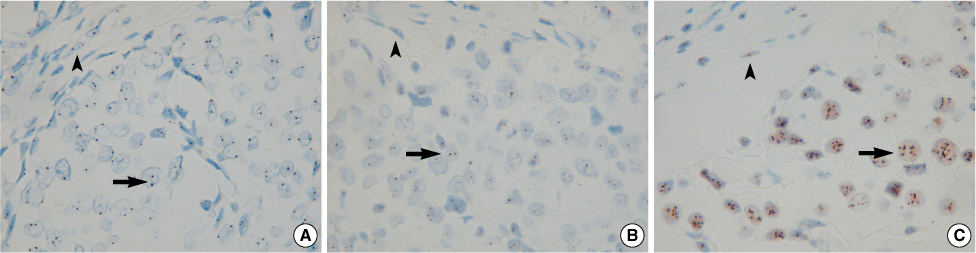
Silver-enhanced in situ hybridization (SISH) is a newly developed method to evaluate HER2 gene amplification in invasive breast carcinomas. Most laboratories widely use fluorescence in situ hybridization (FISH) to evaluate the HER2 gene amplification status because FISH is a very sensitive and accurate technique. However, this technique is not the best because it requires specialized equipment and interpretation skills. We compared a new technique of SISH with FISH for assessing HER2 gene amplification in invasive breast carcinomas. METHODS: HER2 gene amplification was assessed in 165 cases of invasive breast carcinoma by FISH and SISH with constructing a tissue microarray. The tumors were assessed by the guidelines of the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP). Positivity was defined as a HER2/Chromosome 17 ratio greater than 2.2. Negativity was defined if the ratio was less than 1.8. The tumor was considered as equivocal for HER2 gene amplification if the ratio was between 1.8 and 2.2. The HER2 protein status was assessed. Immunostaining for HER2 protein was performed in a Benchmark automatic immunostaining device with using whole tissue sections. RESULTS: There was agreement of the HER2 gene amplification status by SISH and FISH in 162 of 165 cases, which is a concordance rate of 98.2% (kappa=0.94). There were three discrepant cases, with two of them being FISH positive and SISH negative (one case was IHC negative and one case was IHC positive) and one case was FISH negative and SISH equivocal. CONCLUSION: The 98.2% concordance between FISH and SISH meets the ASCO/CAP requirements for test validation of >95% concordance. These results indicate that SISH can be used as an alternative to FISH for assessing the HER2 gene amplification status in breast carcinomas.
MeSH Terms
Figure
Cited by 4 articles
-
Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer
Kyeongmee Park, Sehwan Han, Jung-Yeon Kim, Hyun-Jung Kim, Ji Eun Kwon, Geumhee Gwak
J Breast Cancer. 2011;14(4):276-282. doi: 10.4048/jbc.2011.14.4.276.The Effectiveness of Silver In Situ Hybridization in Patients with Breast Cancer: A Systematic Review
Sunyoung Jang, Seon-Heui Lee, Soojin Kim, You-Kyoung Lee, Young-Hyuck Im, Wonshik Han, Hee-Sook Park
J Breast Cancer. 2011;14(Suppl 1):S1-S9. doi: 10.4048/jbc.2011.14.S.S1.Multiplication of Chromosome 17 Centromere Is Associated with Prognosis in Patients with Invasive Breast Cancers Exhibiting Normal HER2 and TOP2A Status
Aeri Kim, Hyung Chan Shin, Young Kyung Bae, Min Kyoung Kim, Su Hwan Kang, Soo Jung Lee, Eun Hee Lee
J Breast Cancer. 2012;15(1):24-33. doi: 10.4048/jbc.2012.15.1.24.HER2 Status by Standardized Immunohistochemistry and Silver-Enhanced In Situ Hybridization in Korean Breast Cancer
Young Kyung Bae, Gyungyub Gong, Jun Kang, Ahwon Lee, Eun Yoon Cho, Ji Shin Lee, Kwang-Sun Suh, Dong Wha Lee, Woo Hee Jung,
J Breast Cancer. 2012;15(4):381-387. doi: 10.4048/jbc.2012.15.4.381.
Reference
-
1. Sahin AA. Biologic and clinical significance of HER2/neu (cerbB-2) in breast cancer. Adv Anat Pathol. 2000. 7:158–166.
Article2. Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999. 17:2639–2648.
Article3. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001. 344:783–792.
Article4. Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008. 112:533–543.
Article5. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006. 355:2733–2743.
Article6. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.
Article7. Dowsett M, Hanna WM, Kockx M, Penault-Llorca F, Rüschoff J, Gutjahr T, et al. Standardization of HER2 testing: results of an international proficiency-testing ring study. Mod Pathol. 2007. 20:584–591.
Article8. Cell Markers and Cytogenetics Committees College Of American Pathologists. Clinical laboratory assays for HER2/neu amplification and overexpression: quality assurance, standardization, and proficiency testing. Arch Pathol Lab Med. 2002. 126:803–808.9. Dietel M, Ellis IO, Höfler H, Kreipe H, Moch H, Dankof A, et al. Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007. 451:19–25.
Article10. Carbone A, Botti G, Gloghini A, Simone G, Truini M, Curcio MP, et al. Delineation of HER2 gene status in breast carcinoma by silver in situ hybridization is reproducible among laboratories and pathologists. J Mol Diagn. 2008. 10:527–536.
Article11. Pauletti G, Godolphin W, Press MF, Slamon DJ. Detection and quantitation of HER2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene. 1996. 13:63–72.12. Hauser-Kronberger C, Dandachi N. Comparison of chromogenic in situ hybridization with other methodologies for HER2 status assessment in breast cancer. J Mol Histol. 2004. 35:647–653.
Article13. Gupta D, Middleton LP, Whitaker MJ, Abrams J. Comparison of fluorescence and chromogenic in situ hybridization for detection of HER2/neu oncogene in breast cancer. Am J Clin Pathol. 2003. 119:381–387.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Automated Silver-enhanced In Situ Hybridization for Evaluation of HER2 Gene Status in Breast Carcinoma: Comparison with Fluorescence In Situ Hybridization and Immunohistochemistry
- HER2 Status in Gastric Adenocarcinomas Assessed by Immunohistochemistry, Automated Silver-Enhanced In Situ Hybridization and Fluorescence In Situ Hybridization
- Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer
- The Comparison of Automated Silver in situ Hybridization and Fluorescence in situ Hybridization for Evaluating HER2 Gene Amplification in Breast Carcinoma
- HER2-Positive Breast Carcinomas with Co-amplification or Gain of Chromosome 17 Centromere Locus: Report of Three Cases and an Impact on HER2 Testing