J Breast Cancer.
2014 Sep;17(3):287-290. 10.4048/jbc.2014.17.3.287.
Combined Antiangiogenic and Mammalian Target of Rapamycin Inhibitor Targeted Therapy in Metaplastic Breast Cancer Harboring a PIK3CA Mutation
- Affiliations
-
- 1Department of Hematology and Oncology, University of Cincinnati College of Medicine, Cincinnati, USA. agarwari@ucmail.uc.edu
- 2MD Anderson Cancer Center, Houston, USA.
- KMID: 2286357
- DOI: http://doi.org/10.4048/jbc.2014.17.3.287
Abstract
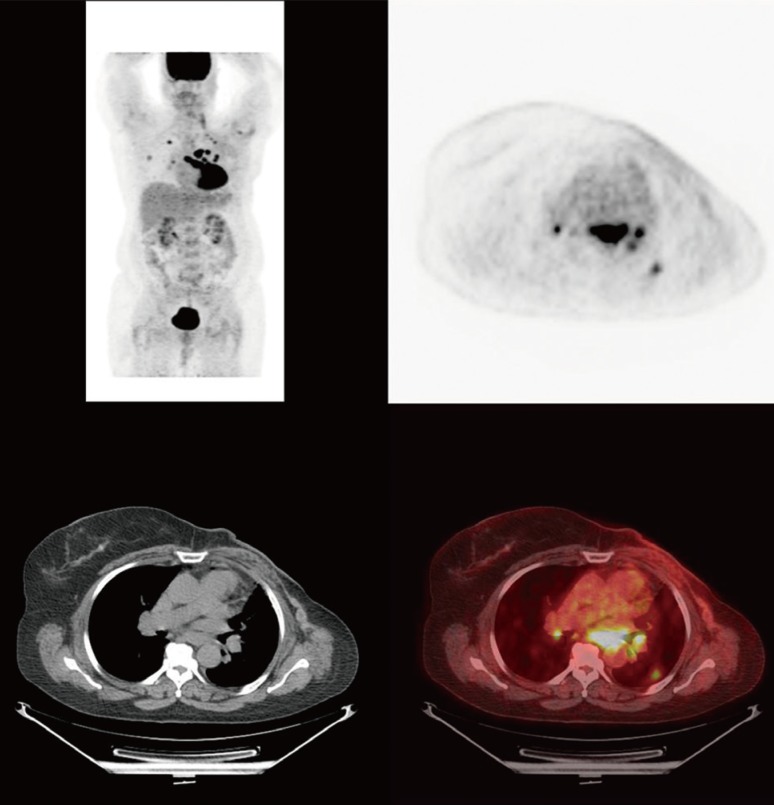
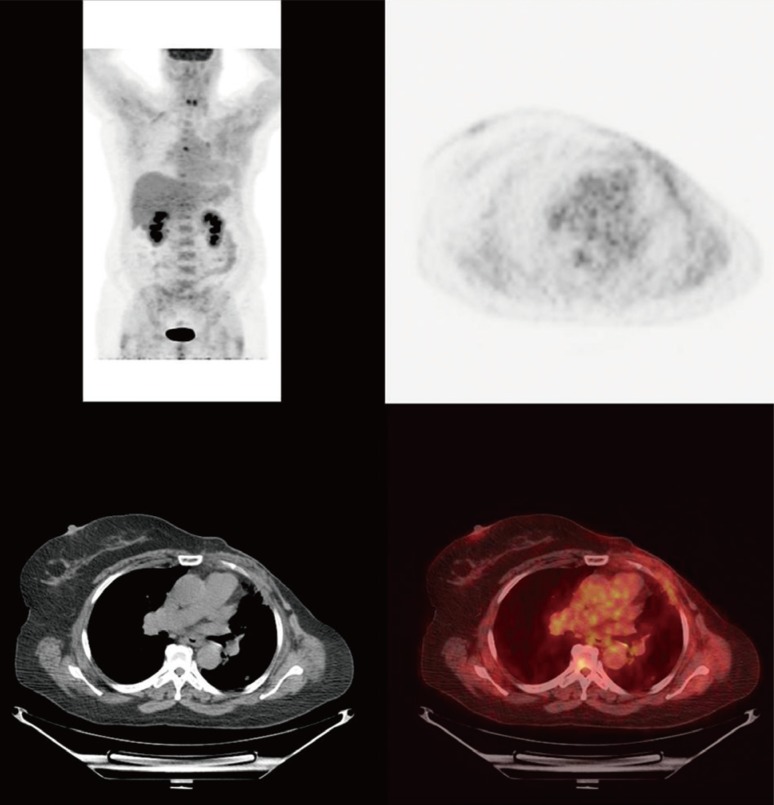
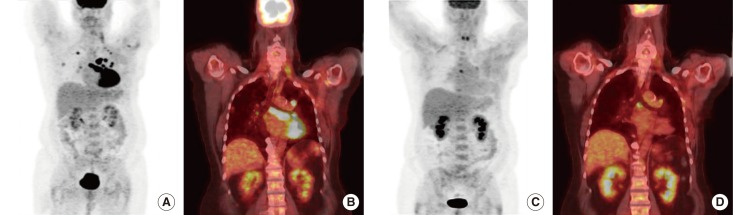
- Metaplastic breast cancer (MpBC) is an extremely rare breast cancer subtype, characterized by a heterogeneous phenotype. MpBC aggressive biology is attributed to its stem cell-like characteristics. Since these tumors are largely chemoresistant, novel targeted therapies should be explored. Herein, we report the clinical course of a 59-year-old African American woman with MpBC with a PIK3CA mutation in codon 545, exon 10 (GAG to AAG; p.Glu545Lys) and a TP53 mutation in codon 286, exon 8 (GAA to AAA; p.Glu286Lys). The same mutations were observed in the primary and secondary sites. The patient was treated with a molecularly matched therapy using a combined antiangiogenic and mammalian target of rapamycin kinase inhibitor strategy that included liposomal doxorubicin, bevacizumab, and temsirolimus. Partial remission was achieved. In this report, the scientific rationale underlying the activity of this combination was explored. In conclusion, patients may benefit from being offered molecular profiling early during the course of the disease to receive a therapy guided accordingly.
MeSH Terms
Figure
Reference
-
1. Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007; 14:166–173. PMID: 17066230.
Article2. Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. 2011; 126:471–478. PMID: 21287362.
Article3. Moulder S, Moroney J, Helgason T, Wheler J, Booser D, Albarracin C, et al. Responses to liposomal Doxorubicin, bevacizumab, and temsirolimus in metaplastic carcinoma of the breast: biologic rationale and implications for stem-cell research in breast cancer. J Clin Oncol. 2011; 29:e572–e575. PMID: 21482991.
Article4. Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009; 69:4116–4124. PMID: 19435916.
Article5. Moroney J, Fu S, Moulder S, Falchook G, Helgason T, Levenback C, et al. Phase I study of the antiangiogenic antibody bevacizumab and the mTOR/hypoxia-inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: tolerance and biological activity. Clin Cancer Res. 2012; 18:5796–5805. PMID: 22927482.
Article6. Nagai MA, Schaer Barbosa H, Zago MA, Araújo Silva W Jr, Nishimoto IN, Salaorni S, et al. TP53 mutations in primary breast carcinomas from white and African-Brazilian patients. Int J Oncol. 2003; 23:189–196. PMID: 12792793.
Article7. Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: prognosis and response to systemic therapy. Ann Oncol. 1999; 10:413–419. PMID: 10370783.
Article8. Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012; 30:777–782. PMID: 22271473.
Article9. Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011; 10:558–565. PMID: 21216929.
Article10. Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003; 9:4641–4652. PMID: 14581333.11. He C, Sun XP, Qiao H, Jiang X, Wang D, Jin X, et al. Downregulating hypoxia-inducible factor-2α improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma. Cancer Sci. 2012; 103:528–534. PMID: 22145922.
Article12. Said R, Hong DS, Wheler JJ, Naing A, Falchook GS, Fu S, et al. p53 mutations in advanced cancers: clinical characteristics and outcomes in a phase I setting. J Clin Oncol. 2012; 30:Abstract #10607.
Article13. Del Bufalo D, Ciuffreda L, Trisciuoglio D, Desideri M, Cognetti F, Zupi G, et al. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006; 66:5549–5554. PMID: 16740688.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biomarkers of Everolimus Sensitivity in Hormone Receptor-Positive Breast Cancer
- Hormone Treatment for Breast Cancer
- PIK3CA Mutation is Associated with Poor Response to HER2-Targeted Therapy in Breast Cancer Patients
- Concomitant PIK3CA and TP53 Mutations in Breast Cancer: An Analysis of Clinicopathologic and Mutational Features, Neoadjuvant Therapeutic Response, and Prognosis
- Improving Conventional or Low Dose Metronomic Chemotherapy with Targeted Antiangiogenic Drugs