Cancer Res Treat.
2023 Apr;55(2):531-541. 10.4143/crt.2022.221.
PIK3CA Mutation is Associated with Poor Response to HER2-Targeted Therapy in Breast Cancer Patients
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea
- 2Department of Breast Surgery, Korea University Anam Hospital, Seoul, Korea
- 3Department of Pathology, Korea University Anam Hospital, Seoul, Korea
- 4Department of Radiology, Korea University Anam Hospital, Seoul, Korea
- 5Department of Radiation Oncology, Korea University Anam Hospital, Seoul, Korea
- KMID: 2541240
- DOI: http://doi.org/10.4143/crt.2022.221
Abstract
- Purpose
Mutations in the PIK3CA gene occur frequently in breast cancer patients. Activating PIK3CA mutations confer resistance to human epidermal growth factor receptor 2 (HER2)-targeted treatments. In this study, we investigated whether PIK3CA mutations were correlated with treatment response or duration in patients with HER2-positive (HER2+) breast cancer.
Materials and Methods
We retrospectively reviewed the clinical information of patients with HER2+ breast cancer who received HER2-targeted therapy for early-stage or metastatic cancers. The pathologic complete response (pCR), progression-free survival (PFS), and overall survival were compared between patients with wild-type PIK3CA (PIK3CAw) and those with mutated PIK3CA (PIK3CAm). Next-generation sequencing was combined with examination of PFS associated with anti-HER2 monoclonal antibody (mAb) treatment.
Results
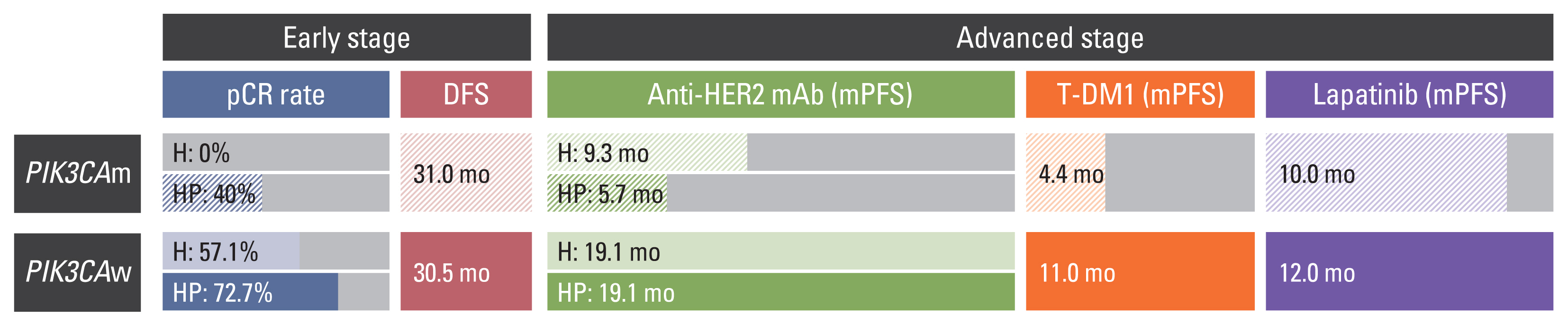
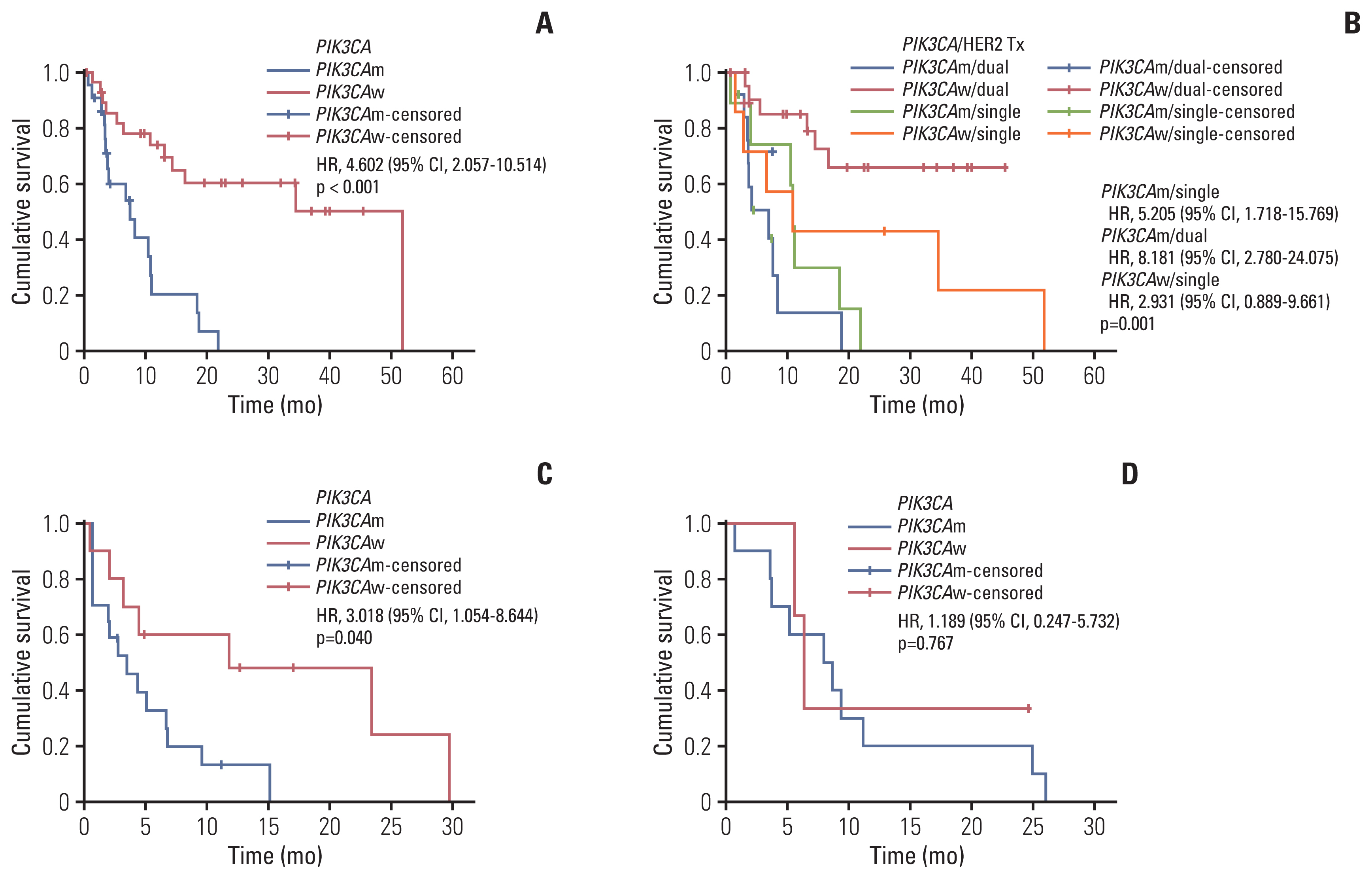
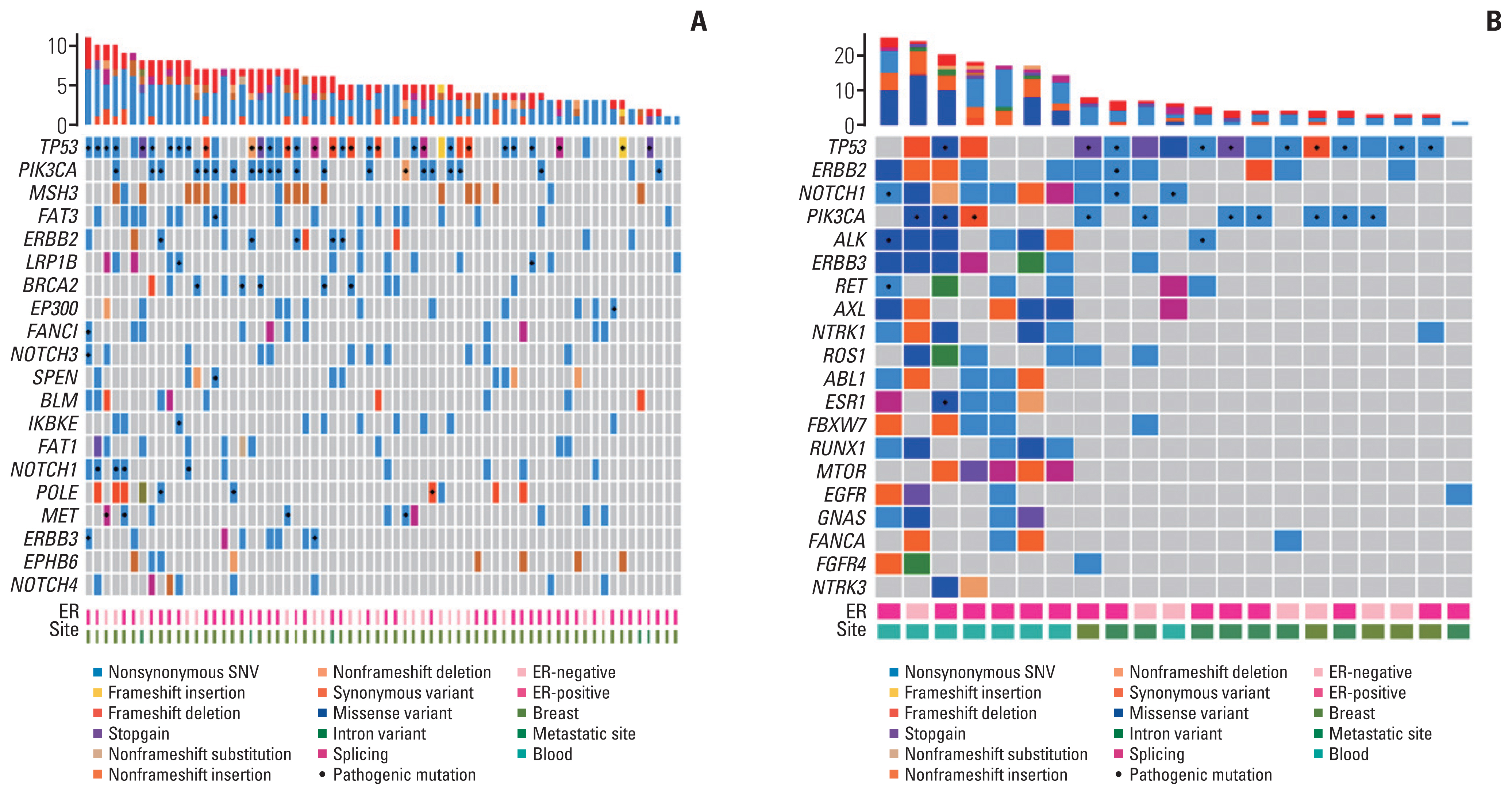
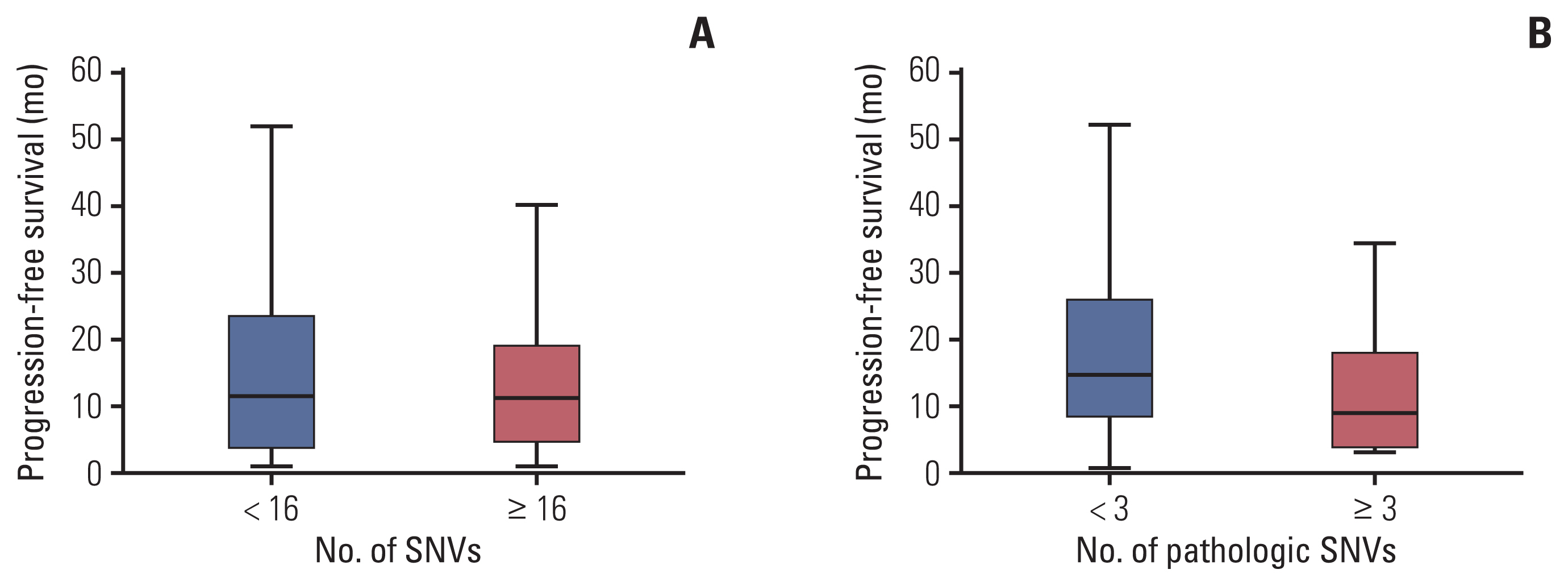
Data from 90 patients with HER2+ breast cancer were analyzed. Overall, 34 (37.8%) patients had pathogenic PIK3CA mutations. The pCR rate of the PIK3CAm group was lower than that of the PIK3CAw group among patients who received neoadjuvant chemotherapy for early-stage cancer. In the metastatic setting, the PIK3CAm group showed a significantly shorter mean PFS (mPFS) with first-line anti-HER2 mAb. The mPFS of second-line T-DM1 was lower in the PIK3CAm group than that in the PIK3CAw group. Sequencing revealed differences in the mutational landscape between PIK3CAm and PIK3CAw tumors.
Conclusion
Patients with HER2+ breast cancer with activating PIK3CA mutations had lower pCR rates and shorter PFS with palliative HER2-targeted therapy than those with wild-type PIK3CA. Precise targeted-therapy is needed to improve survival of patients with HER2+/PIK3CAm breast cancer.
Keyword
Figure
Reference
-
References
1. Gusterson BA, Gelber RD, Goldhirsch A, Price KN, Save-Soderborgh J, Anbazhagan R, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992; 10:1049–56.
Article2. Tsongalis GJ, Ried A Jr. HER2: the neu prognostic marker for breast cancer. Crit Rev Clin Lab Sci. 2001; 38:167–82.
Article3. Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009; 15:7479–91.
Article4. Harbeck N, Huang CS, Hurvitz S, Yeh DC, Shao Z, Im SA, et al. Randomized phase III trial of afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one prior trastuzumab treatment: LUX-Breast 1. Cancer Res. 2015; 75(9 Suppl):P5-19-01.5. Wong HH, Collins J, McAdam K, Wilson C. Trastuzumab beyond progression in advanced breast cancer: national guidance versus oncologist’s decision. Oncology. 2014; 86:22–3.
Article6. National Comprehensive Cancer Network. Breast cancer 2021 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2021. [cited 2021 May 15]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .7. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020; 31:1623–49.8. Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010; 21:255–62.
Article9. Cizkova M, Dujaric ME, Lehmann-Che J, Scott V, Tembo O, Asselain B, et al. Outcome impact of PIK3CA mutations in HER2-positive breast cancer patients treated with trastuzumab. Br J Cancer. 2013; 108:1807–9.
Article10. Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010; 177:1647–56.11. Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011; 17:1116–20.
Article12. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002; 2:489–501.
Article13. Loibl S, Majewski I, Guarneri V, Nekljudova V, Holmes E, Bria E, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol. 2016; 27:1519–25.
Article14. Lee Y, Lee S, Sung JS, Chung HJ, Lim AR, Kim JW, et al. Clinical application of targeted deep sequencing in metastatic colorectal cancer patients: actionable genomic alteration in K-MASTER project. Cancer Res Treat. 2021; 53:123–30.
Article15. Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016; 62:132–7.
Article16. Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000; 6:443–6.
Article17. Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001; 61:4744–9.18. Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002; 62:4132–41.19. Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007; 12:395–402.
Article20. Majewski IJ, Nuciforo P, Mittempergher L, Bosma AJ, Eidtmann H, Holmes E, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015; 33:1334–9.21. Rimawi MF, De Angelis C, Contreras A, Pareja F, Geyer FC, Burke KA, et al. Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer. Breast Cancer Res Treat. 2018; 167:731–40.
Article22. Bianchini G, Kiermaier A, Bianchi GV, Im YH, Pienkowski T, Liu MC, et al. Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res. 2017; 19:16.
Article23. Guarneri V, Dieci MV, Bisagni G, Brandes AA, Frassoldati A, Cavanna L, et al. PIK3CA mutation in the ShortHER Randomized Adjuvant Trial for patients with early HER2(+) breast cancer: association with prognosis and integration with PAM50 subtype. Clin Cancer Res. 2020; 26:5843–51.24. Loi S, Michiels S, Lambrechts D, Fumagalli D, Claes B, Kellokumpu-Lehtinen PL, et al. Somatic mutation profiling and associations with prognosis and trastuzumab benefit in early breast cancer. J Natl Cancer Inst. 2013; 105:960–7.
Article25. Yuan H, Chen J, Liu Y, Ouyang T, Li J, Wang T, et al. Association of PIK3CA mutation status before and after neoadjuvant chemotherapy with response to chemotherapy in women with breast cancer. Clin Cancer Res. 2015; 21:4365–72.26. Kim SB, Do IG, Tsang J, Kim TY, Yap YS, Cornelio G, et al. BioPATH: a biomarker study in Asian patients with HER2+ advanced breast cancer treated with lapatinib and other anti-HER2 therapy. Cancer Res Treat. 2019; 51:1527–39.
Article27. Scaltriti M, Chandarlapaty S, Prudkin L, Aura C, Jimenez J, Angelini PD, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010; 16:2688–95.
Article28. Patra S, Young V, Llewellyn L, Senapati JN, Mathew J. BRAF, KRAS and PIK3CA mutation and sensitivity to trastuzumab in breast cancer cell line model. Asian Pac J Cancer Prev. 2017; 18:2209–13.
Article29. Garay JP, Smith R, Devlin K, Hollern DP, Liby T, Liu M, et al. Sensitivity to targeted therapy differs between HER2-amplified breast cancer cells harboring kinase and helical domain mutations in PIK3CA. Breast Cancer Res. 2021; 23:81.
Article30. Baselga J, Cortes J, Im SA, Clark E, Ross G, Kiermaier A, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014; 32:3753–61.
Article31. Kim SB, Wildiers H, Krop IE, Smitt M, Yu R, Lysbet de Haas S, et al. Relationship between tumor biomarkers and efficacy in TH3RESA, a phase III study of trastuzumab emtansine (T-DM1) vs. treatment of physician’s choice in previously treated HER2-positive advanced breast cancer. Int J Cancer. 2016; 139:2336–42.32. Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008; 112:533–43.
Article33. Martinez-Saez O, Chic N, Pascual T, Adamo B, Vidal M, Gonzalez-Farre B, et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020; 22:45.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concomitant PIK3CA and TP53 Mutations in Breast Cancer: An Analysis of Clinicopathologic and Mutational Features, Neoadjuvant Therapeutic Response, and Prognosis
- PIK3CA Mutations and Neoadjuvant Therapy Outcome in Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: A Sequential Analysis
- Targeted Therapy for Breast Cancer
- Prevalence and Prognostic Role of PIK3CA/AKT1 Mutations in Chinese Breast Cancer Patients
- Prognostic Value of the Evolution of HER2-Low Expression after Neoadjuvant Chemotherapy