Cancer Res Treat.
2023 Oct;55(4):1210-1221. 10.4143/crt.2022.1633.
Prognostic Value of the Evolution of HER2-Low Expression after Neoadjuvant Chemotherapy
- Affiliations
-
- 1Department of Breast Disease, Henan Breast Cancer Center, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
- KMID: 2547795
- DOI: http://doi.org/10.4143/crt.2022.1633
Abstract
- Purpose
Patients with human epidermal growth factor receptor 2 (HER2)–low advanced breast cancer can benefit from trastuzumab deruxtecan. Given the unclear prognostic characteristics of HER2-low breast cancer, we investigated the prognostic characteristics of HER2-low expression from primary tumor to residual disease after neoadjuvant chemotherapy (NACT).
Materials and Methods
The data of HER2-negative patients receiving NACT at our center were collected. Pathological complete response (pCR) rate were compared between HER2-0 and HER2-low patients. The evolution of HER2 expression from primary tumor to residual disease and its impact on disease-free survival (DFS) were examined.
Results
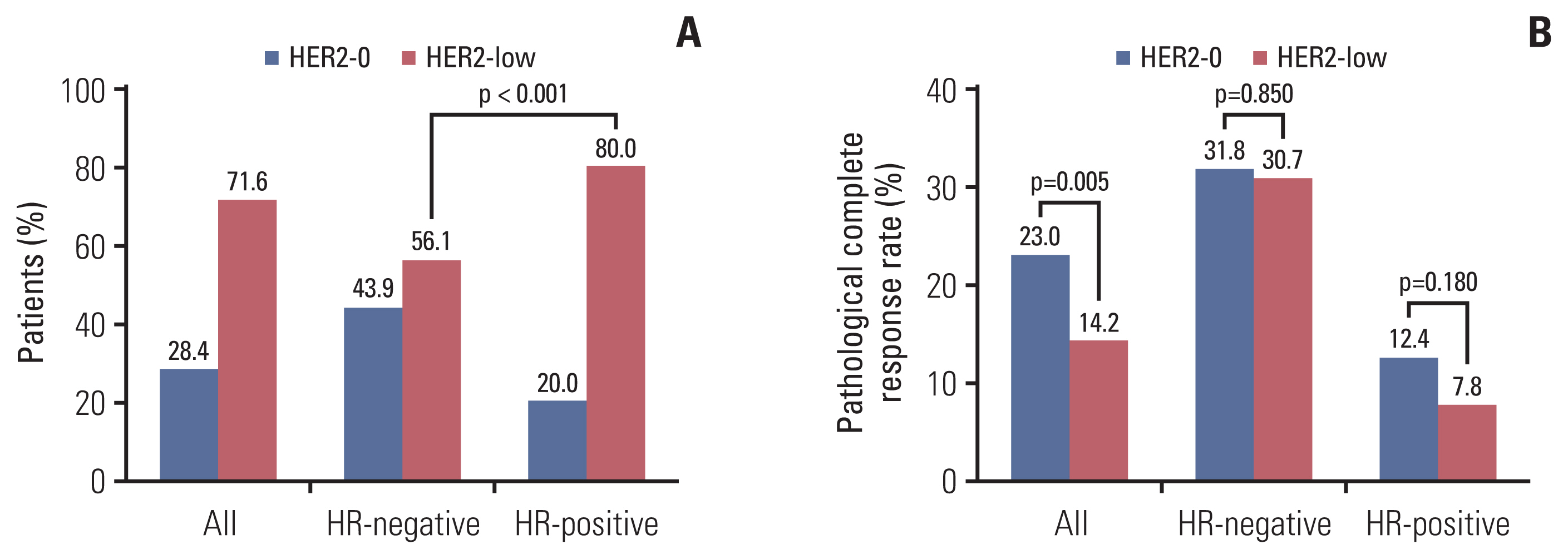
Of the 690 patients, 494 patients had HER2-low status, of which 72.3% were hormone receptor (HR)–positive (p < 0.001). The pCR rates of HER2-low and HER2-0 patients (14.2% vs. 23.0%) showed no difference in multivariate analysis regardless of HR status. No association was observed between DFS and HER2 status. Of the 564 non-pCR patients, 57 (10.1%) changed to HER2-positive, and 64 of the 150 patients (42.7%) with HER2-0 tumors changed to HER2-low. HER2-low (p=0.004) and HR-positive (p=0.010) tumors before NACT were prone to HER2 gain. HER2 gain patients had a better DFS compared with HER2-negative maintained patients (87.9% vs. 79.5%, p=0.048), and the DFS of targeted therapy group was better than that of no targeted therapy group (92.4% vs. 66.7%, p=0.016).
Conclusion
Although HER2-low did not affect the pCR rate and DFS, significant evolution of HER2-low expression after NACT creates opportunities for targeted therapy including trastuzumab.
Keyword
Figure
Cited by 1 articles
-
HER2-Low Breast Cancer: Now and in the Future
Sora Kang, Sung-Bae Kim
Cancer Res Treat. 2024;56(3):700-720. doi: 10.4143/crt.2023.1138.
Reference
-
References
1. Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018; 36:2105–22.2. Piccart M, Procter M, Fumagalli D, de Azambuja E, Clark E, Ewer MS, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021; 39:1448–57.3. Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020; 21:519–30.4. Fehrenbacher L, Cecchini RS, Geyer CE Jr, Rastogi P, Costantino JP, Atkins JN, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020; 38:444–53.5. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020; 38:1951–62.6. Schalper KA, Kumar S, Hui P, Rimm DL, Gershkovich P. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria. Arch Pathol Lab Med. 2014; 138:213–9.7. Banerji U, van Herpen CM, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019; 20:1124–35.8. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020; 38:1887–96.9. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022; 387:9–20.10. Schettini F, Chic N, Braso-Maristany F, Pare L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021; 7:1.11. Zhang G, Ren C, Li C, Wang Y, Chen B, Wen L, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022; 20:142.12. Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021; 22:1151–61.13. Tarantino P, Jin Q, Tayob N, Jeselsohn RM, Schnitt SJ, Vincuilla J, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. 2022; 8:1177–83.14. Bao KK, Sutanto L, Tse SS, Man Cheung K, Chan JC. The Association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor-positive, ERBB2-negative metastatic breast cancer. JAMA Netw Open. 2021; 4:e2133132.15. de Nonneville A, Houvenaeghel G, Cohen M, Sabiani L, Bannier M, Viret F, et al. Pathological complete response rate and disease-free survival after neoadjuvant chemotherapy in patients with HER2-low and HER2-0 breast cancers. Eur J Cancer. 2022; 176:181–8.16. Gahlaut R, Bennett A, Fatayer H, Dall BJ, Sharma N, Velikova G, et al. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression: implications for the practising oncologist. Eur J Cancer. 2016; 60:40–8.17. Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K, et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol. 2016; 27:480–7.18. Miglietta F, Griguolo G, Bottosso M, Giarratano T, Lo Mele M, Fassan M, et al. HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer. 2022; 8:66.19. Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK, et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022; 24:22.20. Horisawa N, Adachi Y, Takatsuka D, Nozawa K, Endo Y, Ozaki Y, et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer. 2022; 29:234–41.21. Fernandez AI, Liu M, Bellizzi A, Brock J, Fadare O, Hanley K, et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 2022; 8:1–4.22. Villegas SL, Nekljudova V, Pfarr N, Engel J, Untch M, Schrodi S, et al. Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors: an analysis of 2765 patients from neoadjuvant clinical trials. Eur J Cancer. 2021; 148:159–70.23. Kang S, Lee SH, Lee HJ, Jeong H, Jeong JH, Kim JE, et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J Cancer. 2022; 176:30–40.24. Guven DC, Kaya MB, Fedai B, Ozden M, Yildirim HC, Kosemehmetoglu K, et al. HER2-low breast cancer could be associated with an increased risk of brain metastasis. Int J Clin Oncol. 2022; 27:332–9.25. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011; 62:233–47.26. Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008; 29:217–33.27. Mutai R, Barkan T, Moore A, Sarfaty M, Shochat T, Yerushalmi R, et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021; 60:62–9.28. Almstedt K, Heimes AS, Kappenberg F, Battista MJ, Lehr HA, Krajnak S, et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer. 2022; 173:10–9.29. Tarantino P, Gandini S, Nicolo E, Trillo P, Giugliano F, Zagami P, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer. 2022; 163:35–43.30. Miglietta F, Griguolo G, Bottosso M, Giarratano T, Lo Mele M, Fassan M, et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer. 2021; 7:137.31. Yoshida A, Hayashi N, Suzuki K, Takimoto M, Nakamura S, Yamauchi H. Change in HER2 status after neoadjuvant chemotherapy and the prognostic impact in patients with primary breast cancer. J Surg Oncol. 2017; 116:1021–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of HER2-Low Status on Pathologic Complete Response and Survival Outcome Among Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy
- HER2-negative or low expression as an unfavorable prognostic factor in patients with stage I/II uterine carcinosarcoma
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- The Predictive Value of Serum HER2/neu for Response to Anthracycline-Based and Trastuzumab-Based Neoadjuvant Chemotherapy
- The Effect of Neoadjuvant Chemotherapy on the Biological Prognostic Markers in Breast Cancer Patients