Cancer Res Treat.
2007 Dec;39(4):150-159.
Improving Conventional or Low Dose Metronomic Chemotherapy with Targeted Antiangiogenic Drugs
- Affiliations
-
- 1Sunnybrook Research Institute, Sunnybrook Health Sciences Centre and the University of Toronto, Toronto, Canada. Robert.kerbel@sri.utoronto.ca
Abstract
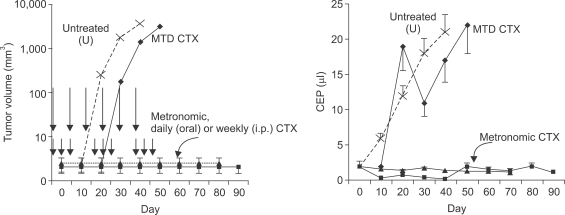
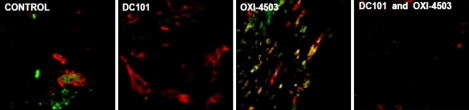
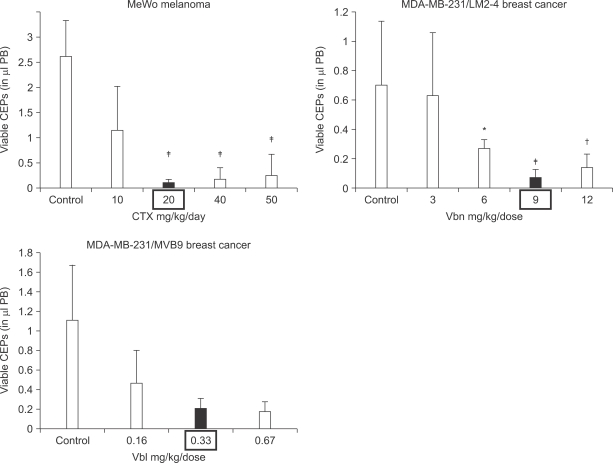
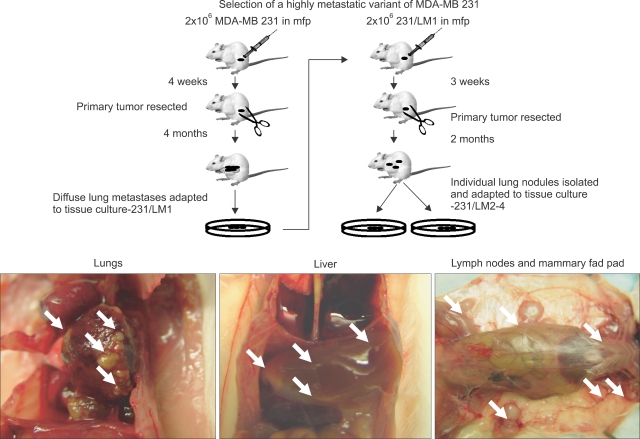
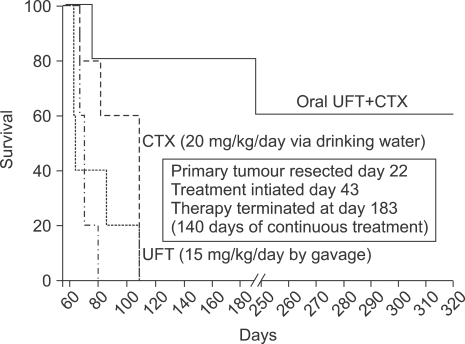
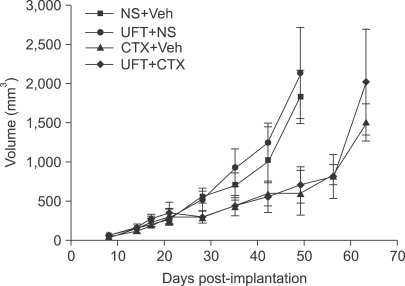
- One of the most significant developments in medical oncology practice has been the approval of various antiangiogenic drugs for the treatment of a number of different malignancies. These drugs include bevacizumab (Avastin(R)), the anti-VEGF monoclonal antibody. Thus far, bevacizumab appears to induce clinical benefit in patients who have advanced metastatic disease only or primarily when it is combined with conventional chemotherapy. The reasons for the chemo-enhancing effects of bevacizumab are unknown, and this is a subject that we have been actively studying along with additional ways that antiangiogenic drugs may be combined with chemotherapy. In this respect, we have focused much of our effort on metronomic low dose chemotherapy. We have been studying the hypothesis that some chemotherapy drugs at maximum tolerated doses or other cytotoxic-like drugs such as acute "vascular disrupting agents" (VDAs) can cause an acute mobilization of proangiogenic cells from the bone marrow which home to and colonize the treated tumors, thus accelerating their recovery. These cells include endothelial progenitor cells. This systemic process can be largely blocked by a targeted antiangiogenic drug, e.g. anti-VEGFR-2 antibodies. In addition, metronomic chemotherapy, i.e., close regular administration of chemotherapy drugs at low non-toxic doses with no breaks, over prolonged periods of time not only prevents the acute CEP bone marrow response, but can even target the cells. This potential antiangiogenic effect of metronomic chemotherapy can also be boosted by combination with a targeted antiangiogenic agent. Treatment combinations of metronomic chemotherapy and an antiangiogenic drug have moved into phase II clinical trial testing with particularly encouraging results thus far reported in metastatic breast and recurrent ovarian cancer. Oral chemotherapy drugs such as cyclophosphamide (CTX), methotrexate are the main chemotherapeutics used for such trials. Oral 5-FU prodrugs such as UFT would also appear to be highly suitable based on long term adjuvant therapy studies in patients. Recent preclinical results using metronomic cyclophosphamide and metronomic UFT in models of advanced metastatic breast cancer suggest that this type of combination might be particularly promising for metronomic chemotherapy in this indication, particularly when combined with a targeted antiangiogenic drug.
Keyword
MeSH Terms
-
Antibodies
Bevacizumab
Bone Marrow
Breast
Breast Neoplasms
Colon
Cyclophosphamide
Drug Therapy*
Fluorouracil
Humans
Maximum Tolerated Dose
Medical Oncology
Methotrexate
Neoplasm Metastasis
Ovarian Neoplasms
Prodrugs
Stem Cells
Vascular Endothelial Growth Factor A
Antibodies
Cyclophosphamide
Fluorouracil
Methotrexate
Prodrugs
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005; 438:967–974. PMID: 16355214.
Article2. Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006; 312:1171–1175. PMID: 16728631.
Article3. Kerbel RS, Kamen BA. Antiangiogenic basis of low-dose metronomic chemotherapy. Nature Rev Cancer. 2004; 4:423–436. PMID: 15170445.4. Miller KD, Sweeney CJ, Sledge GW Jr. Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol. 2001; 19:1195–1206. PMID: 11181686.
Article5. Browder T, Butterfield CE, Kraling BM, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000; 60:1878–1886. PMID: 10766175.6. Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, et al. Low dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002; 13:73–80. PMID: 11863115.7. Emmenegger U, Man S, Shaked Y, Francia G, Wong JW, Hicklin DJ, et al. A comparative analysis of low dose metronomic cyclophosphamide reveals absent or low grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res. 2004; 64:3994–4000. PMID: 15173013.8. Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin D, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000; 105:R15–R24. PMID: 10772661.
Article9. Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose "chemo-switch" regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005; 23:939–952. PMID: 15557593.
Article10. du Manoir JM, Francia G, Man S, Mossoba M, Medin JA, Viloria-Petit A, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab (Herceptin®) in human breast cancer xenografts. Clin Cancer Res. 2006; 12:904–916. PMID: 16467105.11. Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003; 63:8408–8413. PMID: 14679003.12. Bottini A, Generali D, Brizzi MP, Fox SB, Bersiga A, Bonardi S, et al. Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006; 24:3623–3628. PMID: 16877730.
Article13. Bocci G, Tuccori M, Emmenegger U, Liguori V, Kerbel RS, Del Tacca M. Cyclophosphamide-methotrexate "metronomic" chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol. 2004; 16:1243–1252. PMID: 15905308.
Article14. Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, et al. A randomized trial of adjuvant chemotherapy with uraciltegafur for adenocarcinoma of the lung. N Engl J Med. 2004; 350:1713–1721. PMID: 15102997.
Article15. Tannock IF. Population kinetics of carcinoma cells, capillary endothelial cells, and fibroblasts in a transplanted mouse mammary tumor. Cancer Res. 1970; 30:2470–2476. PMID: 4097429.16. Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000; 60:1388–1393. PMID: 10728704.17. Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991; 13:31–36. PMID: 1722975.
Article18. Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003; 63:4342–4346. PMID: 12907602.19. Furstenberger G, von Moos R, Lucas R, Thurlimann B, Senn HJ, Hamacher J, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy or primary breast cancer. Br J Cancer. 2006; 94:524–531. PMID: 16450002.20. Hudis CA. Clinical implications of antiangiogenic therapies. Oncology. 2005; 19:26–31. PMID: 15934500.21. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275:964–967. PMID: 9020076.
Article22. Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis: implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005; 7:101–111. PMID: 15652753.23. Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: from promiscuity to surrogate marker and target identification. Nature Rev Cancer. 2006; 6:835–845. PMID: 17036040.24. Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003; 300:1155–1159. PMID: 12750523.
Article25. Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005; 102:18111–18116. PMID: 16326806.
Article26. Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006; 313:1785–1787. PMID: 16990548.
Article27. Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005; 5:423–435. PMID: 15928673.
Article28. Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4(+)CD25(+) regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2006; 56:641–648. PMID: 16960692.
Article29. Shaked Y, Emmengger U, Man S, Cervi D, Bertolini F, Ben-David Y, et al. The optimal biological dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005; 106:3058–3061. PMID: 15998832.30. Ng SS, Sparreboom A, Shaked Y, Lee C, Man S, Desai N, et al. Influence of formulation vehicle on metronomic taxane chemotherapy: albumin-bound versus cremophor EL-based paclitaxel. Clin Cancer Res. 2006; 12:4331–4338. PMID: 16857808.
Article31. Munoz R, Man S, Shaked Y, Lee C, Wong J, Francia G, et al. Highly efficacious non-toxic treatment for advanced metastatic breast cancer using combination UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006; 66:3386–3391. PMID: 16585158.32. Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, et al. Circulating endothelial cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006; 108:452–459. PMID: 16543470.33. Man S, Bocci G, Francia G, Green S, Jothy S, Bergers G, et al. Antitumor and anti-angiogenic effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002; 62:2731–2735. PMID: 12019144.34. Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived - but they can be improved. Cancer Biol Ther. 2003; 2:S134–S139. PMID: 14508091.35. Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007; epub ahead of print.
Article36. Rocca A, Dellapasqua S, Pietri E. Metronomic chemotherapy with capecitabine and oral cyclophosphamide in combination with bevacizumab in metastatic breast cancer (mbc): evidence of activity of an antianigogenic treatment. Proc Am Soc Clin Oncol. 2007; #11501(2007) (Abstract).37. Buckstein R, Crump M, Shaked Y, Nayar R, Foden C, Turner R, et al. High dose celecoxib and metronomic 'low dose' cyclophosphamide is effective and safe therapy in patients with relapsed and refractory aggressive histology NHL. Clin Cancer Res. 2006; 12:5190–5198. PMID: 16951238.38. Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low dose metronomic oral cyclophosphamide in recurrent ovarian cancer. A study of the California, Chicago and Princess Margaret Hospital Phase II Consortia. J Clin Oncol. 2007; in press.39. Glode LM, Crighton F, Barqawi A, Kerbel RS, Berman C, Crawford D. Metronomic therapy with cyclophosphamide and dexamethasone for prostate cancer. Cancer. 2003; 98:1643–1648. PMID: 14534880.40. Orlando L, Cardillo A, Rocca A, Balduzzi A, Ghisini R, Peruzzotti G, et al. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2006; 17:961–967. PMID: 16940806.
Article41. Vogt T, Hafner C, Bross K, Bataille F, Jauch KW, Berand A, et al. Antiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumors. Cancer. 2003; 98:2251–2256. PMID: 14601096.
Article42. Kong DS, Lee JI, Kim WS, Son MJ, Lim dH, Kim ST, et al. A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma. Oncol Rep. 2006; 16:1117–1121. PMID: 17016602.
Article43. Reichle A, Bross K, Vogt T, Bataille F, Wild P, Berand A, et al. Pioglitazone and rofecoxib combined with angiostatically scheduled trofosfamide in the treatment of far-advanced melanoma and soft tissue sarcoma. Cancer. 2004; 101:2247–2256. PMID: 15470711.
Article44. Buckstein R, Kerbel RS, Shaked Y, Nayar R, Foden C, Turner R, et al. High-dose celecoxib and metronomic "low-dose" cyclophosphamide is an effective and safe therapy in patients with relapsed and refractory aggressive histology non-Hodgkin's lymphoma. Clin Cancer Res. 2006; 12:5190–5198. PMID: 16951238.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two cases of advanced hepatocellular carcinoma achieving a complete response with metronomic chemotherapy via the hepatic artery
- Can metronomic chemotherapy be an alternative to sorafenib in advanced hepatocellular carcinoma?
- Advanced ovarian cancer: what should be the standard of care?
- Efficacy and safety of metronomic chemotherapy for patients with advanced primary hepatocellular carcinoma with major portal vein tumor thrombosis
- Commentary on “Metronomic S-1 Adjuvant Chemotherapy Improves Survival in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma”