J Breast Cancer.
2014 Sep;17(3):219-225. 10.4048/jbc.2014.17.3.219.
Antiproliferatory Effects of Crab Shell Extract on Breast Cancer Cell Line (MCF7)
- Affiliations
-
- 1Fertility and Infertility Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran. mkhazaei1345@yahoo.com
- KMID: 2286348
- DOI: http://doi.org/10.4048/jbc.2014.17.3.219
Abstract
- PURPOSE
Breast cancer is the most common type of cancer in women. Despite various pharmacological developments, the identification of new therapies is still required for treating breast cancer. Crab is often recommended as a traditional medicine for cancer. This study aimed to determine the in vitro effect of a hydroalcoholic crab shell extract on a breast cancer cell line.
METHODS
In this experimental study, MCF7 breast cancer cell line was used. Crab shell was powdered and a hydroalcoholic (70degrees ethanol) extract was prepared. Five concentrations (100, 200, 400, 800, and 1,000 microg/mL) were added to the cells for three periods, 24, 48, and 72 hours. The viability of the cells were evaluated using trypan blue and 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays. Cell apoptosis was determined using the terminal deoxynucleotidyl transferase dUTP nick end labeling method. Nitric oxide (NO) level was assessed using the Griess method. Data were analyzed using analysis of variance, and p<0.05 was considered significant.
RESULTS
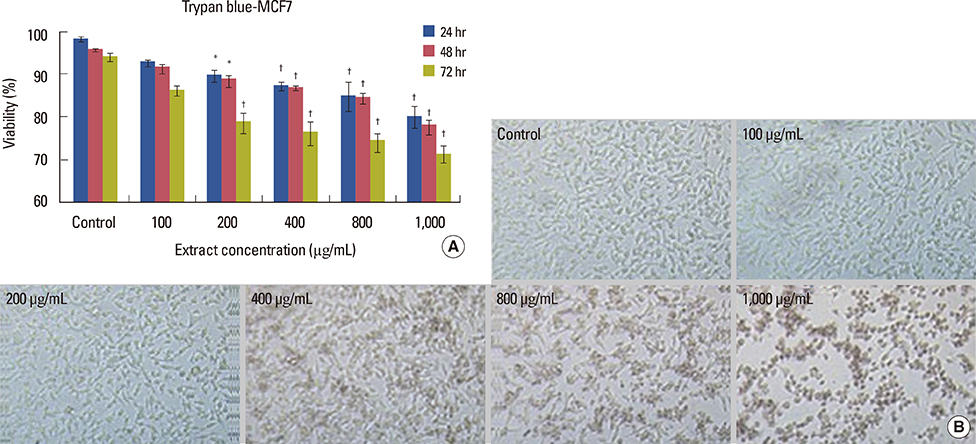
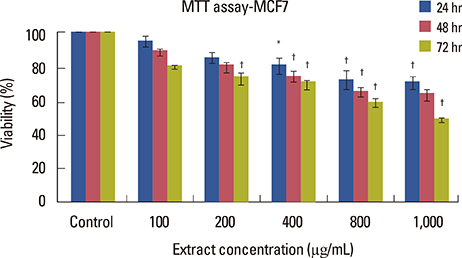
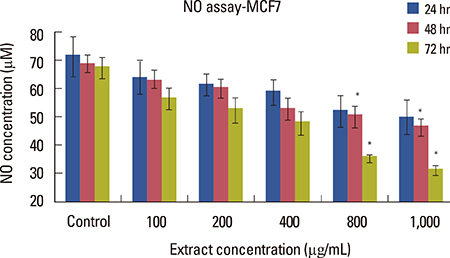
Cell viability decreased depending on dose and time, and was significantly different in the groups that were treated with 400, 800, and 1,000 microg/mL doses compared to that in the control group (p<0.001). Increasing the dose significantly increased apoptosis (p<0.001). NO secretion from MCF7 cells significantly decreased in response to different concentrations of the extract in a dose- and time-dependent manner (p<0.050).
CONCLUSION
The crab shell extract inhibited the proliferation of MCF7 cells by increasing apoptosis and decreasing NO production.
Keyword
MeSH Terms
Figure
Reference
-
1. Rani D, Somasundaram VH, Nair S, Koyakutty M. Advances in cancer nanomedicine. J Indian Inst Sci. 2012; 92:187–218.2. Curado MP. Breast cancer in the world: incidence and mortality. Salud Publica Mex. 2011; 53:372–384.3. Kateb B, Chiu K, Black KL, Yamamoto V, Khalsa B, Ljubimova JY, et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: what should be the policy? Neuroimage. 2011; 54:Suppl 1. S106–S124.
Article4. Grever MC. Cancer drug discovery and development. In : De Vita VH, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott-Raven;2001. p. 328–339.5. Domard A, Domard M. Chitosan: structure-properties relationship and biomedical applications. In : Dumitriu S, editor. Polymeric Biomaterials. 2nd ed. New York: Marcel Dekker Inc.;2002. p. 187–212.6. Acosta N, Jiménez C, Borau V, Heras A. Extraction and characterization of chitin from crustaceans. Biomass Bioenergy. 1993; 5:145–153.
Article7. Jeon YJ, Kim SK. Antitumor activity of chitosan oligosaccharides produced in ultrafiltration membrane reactor system. J Microbiol Biotechnol. 2002; 12:503–507.8. Tsukada K, Matsumoto T, Aizawa K, Tokoro A, Naruse R, Suzuki S, et al. Antimetastatic and growth-inhibitory effects of N-acetylchitohexaose in mice bearing Lewis lung carcinoma. Jpn J Cancer Res. 1990; 81:259–265.
Article9. Maeda Y, Kimura Y. Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. J Nutr. 2004; 134:945–950.
Article10. Huang R, Mendis E, Rajapakse N, Kim SK. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006; 78:2399–2408.
Article11. Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012; 17:3202–3242.
Article12. Nishino H, Murakosh M, Ii T, Takemura M, Kuchide M, Kanazawa M, et al. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002; 21:257–264.13. Sachindra NM, Bhaskar N, Mahendrakar NS. Carotenoids in crabs from marine and fresh waters of India. Lebenson Wiss Technol. 2005; 38:221–225.
Article14. Brozmanová J, Mániková D, Vlčková V, Chovanec M. Selenium: a double-edged sword for defense and offence in cancer. Arch Toxicol. 2010; 84:919–938.
Article15. Whanger PD. Selenium and its relationship to cancer: an update. Br J Nutr. 2004; 91:11–28.
Article16. Ghosh K, Chandra K, Ojha AK, Sarkar S, Islam SS. Structural identification and cytotoxic activity of a polysaccharide from the fruits of Lagenaria siceraria (Lau). Carbohydr Res. 2009; 344:693–698.
Article17. Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001; 5:62–71.
Article18. Chen T, Wong YS. Selenocystine induces caspase-independent apoptosis in MCF-7 human breast carcinoma cells with involvement of p53 phosphorylation and reactive oxygen species generation. Int J Biochem Cell Biol. 2009; 41:666–676.
Article19. Shah YM, Kaul A, Dong Y, Ip C, Rowan BG. Attenuation of estrogen receptor alpha (ERalpha) signaling by selenium in breast cancer cells via downregulation of ERalpha gene expression. Breast Cancer Res Treat. 2005; 92:239–250.
Article20. Shen KT, Chen MH, Chan HY, Jeng JH, Wang YJ. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem Toxicol. 2009; 47:1864–1871.
Article21. Nakahara S, Miura Y, Yagasaki K. Inhibitory action of carotenoids in crab shell on the invasion of hepatoma cells co-cultured with mesothelial cells. European Society of Animal Cell Technology. Japanese Association for Animal Cell Technology. Animal Cell Technology: Challenges for the 21st Century. Boston: Kluwer;2002. p. 409–413.22. Yin MB, Li ZR, Tóth K, Cao S, Durrani FA, Hapke G, et al. Potentiation of irinotecan sensitivity by Se-methylselenocysteine in an in vivo tumor model is associated with downregulation of cyclooxygenase-2, inducible nitric oxide synthase, and hypoxia-inducible factor 1alpha expression, resulting in reduced angiogenesis. Oncogene. 2006; 25:2509–2519.
Article23. Parizadeh MR, Gharib FG, Abbaspour AR, Afshar JT, Ghayour-Mobarhan M. Effects of aqueous saffron extract on nitric oxide production by two human carcinoma cell lines: hepatocellular carcinoma (HepG2) and laryngeal carcinoma (Hep2). Avicenna J Phytomed. 2011; 1:43–50.24. Gasparian AV, Yao YJ, Lü J, Yemelyanov AY, Lyakh LA, Slaga TJ, et al. Selenium compounds inhibit I kappa B kinase (IKK) and nuclear factor-kappa B (NF-kappa B) in prostate cancer cells. Mol Cancer Ther. 2002; 1:1079–1087.25. Kuppusamy S, Karuppaiah J. Antioxidant and cytotoxic efficacy of chitosan on bladder cancer. Asian Pac J Trop Dis. 2012; 2:Suppl 2. S769–S773.
Article26. Lim SA, Lee JY, Jung WH, Lim EH, Joo MK, Lee BJ, et al. Anticancer effects of astaxanthin and alpha-tocopherol in esophageal cancer cell lines. Korean J Helicobacter Up Gastrointest Res. 2011; 11:170–175.
Article27. Palozza P, Torelli C, Boninsegna A, Simone R, Catalano A, Mele MC, et al. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009; 283:108–117.
Article28. Imbalzano KM, Tatarkova I, Imbalzano AN, Nickerson JA. Increasingly transformed MCF-10A cells have a progressively tumor-like phenotype in three-dimensional basement membrane culture. Cancer Cell Int. 2009; 9:7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Selective B-RAF V600E Inhibitor PLX4032 Inhibits the Growth of Breast Cancer Cell Lines through Cell Cycle Arrest
- Expression of Cell Cycle Regulatory Proteins on the Solid Tumor Cell Line After Irradiation Under Hypoxia
- Adenovirus adenine nucleotide translocator-2 shRNA effectively induces apoptosis and enhances chemosensitivity by the down-regulation of ABCG2 in breast cancer stem-like cells
- Loquat (Eriobotrya japonica) extracts suppress the adhesion, migration and invasion of human breast cancer cell line
- Overexpression of CD44 Standard Isoform Upregulates HIF-1α Signaling in Hypoxic Breast Cancer Cells