Korean J Physiol Pharmacol.
2015 Sep;19(5):467-472. 10.4196/kjpp.2015.19.5.467.
Trichostatin A Modulates Angiotensin II-induced Vasoconstriction and Blood Pressure Via Inhibition of p66shc Activation
- Affiliations
-
- 1Research Institute for Medical Sciences, Department of Physiology, School of Medicine, Chungnam National University, Daejeon 301-747, Korea. bhjeon@cnu.ac.kr
- KMID: 2285592
- DOI: http://doi.org/10.4196/kjpp.2015.19.5.467
Abstract
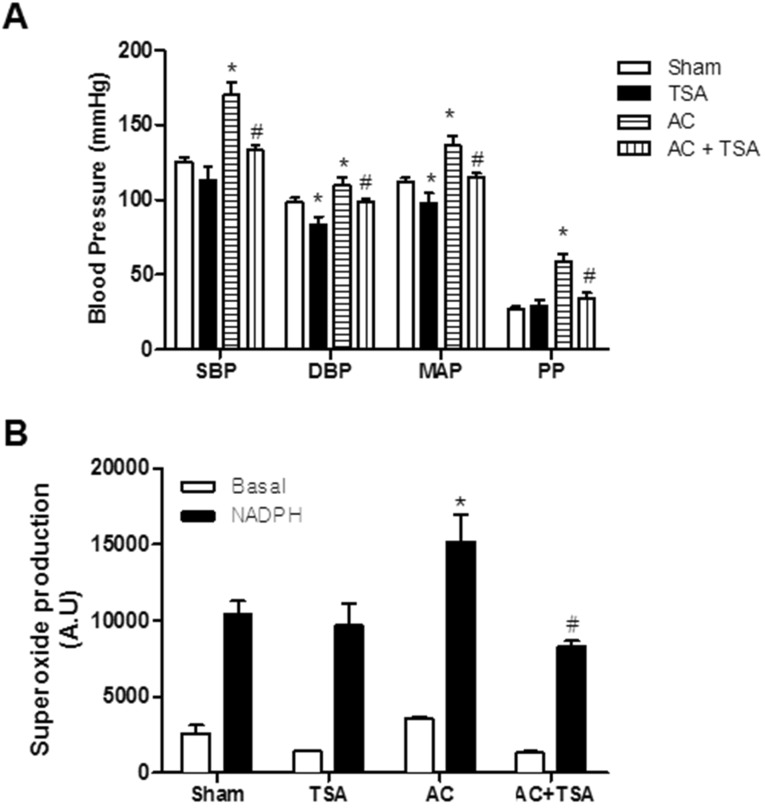
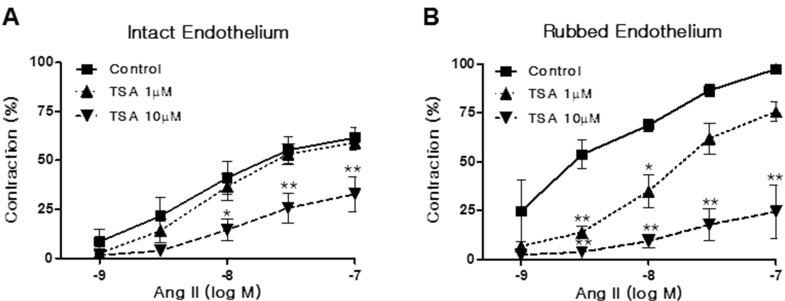
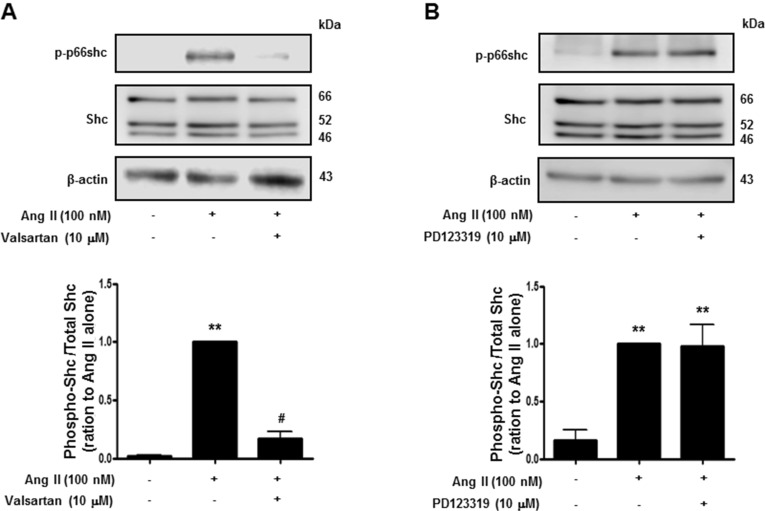
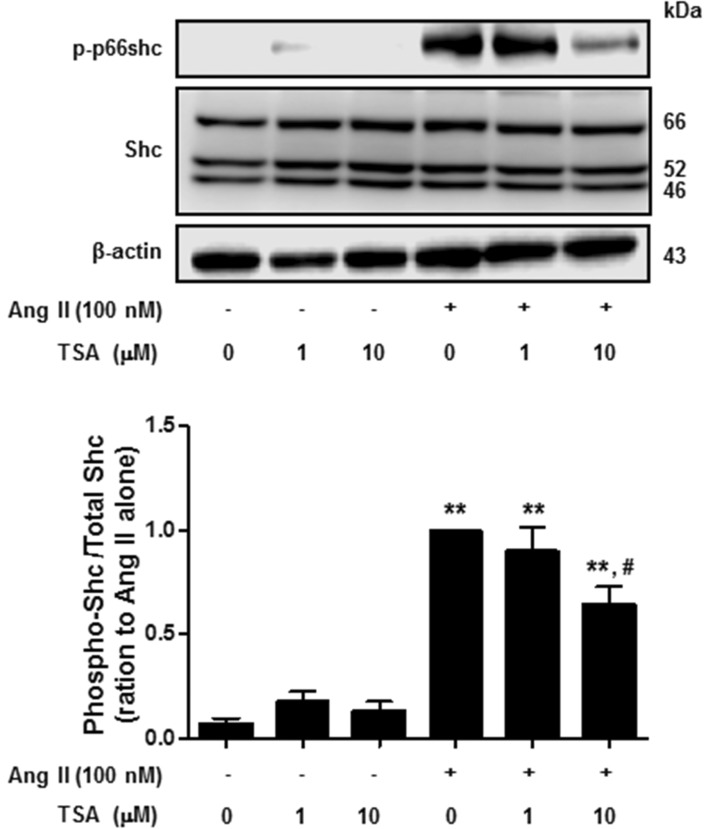
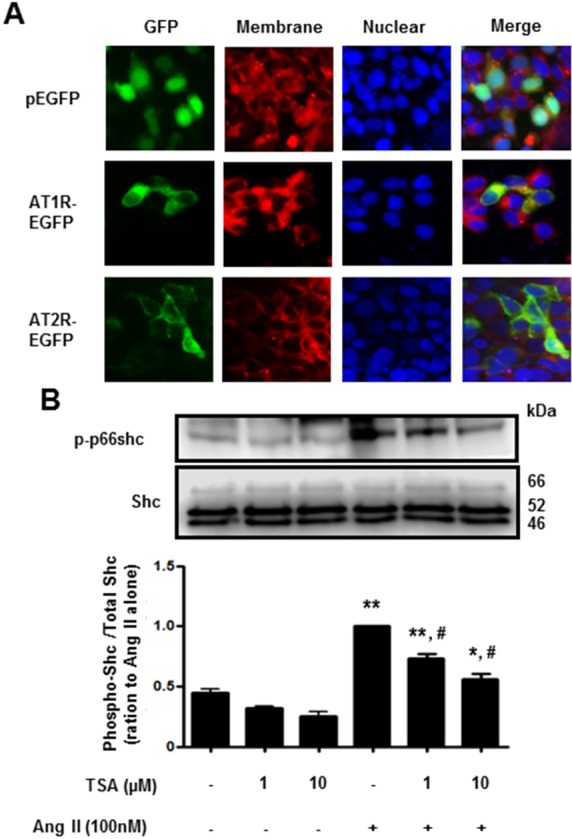
- Histone deacetylase (HDAC) has been recognized as a potentially useful therapeutic target for cardiovascular disorders. However, the effect of the HDAC inhibitor, trichostatin A (TSA), on vasoreactivity and hypertension remains unknown. We performed aortic coarctation at the inter-renal level in rats in order to create a hypertensive rat model. Hypertension induced by abdominal aortic coarctation was significantly suppressed by chronic treatment with TSA (0.5 mg/kg/day for 7 days). Nicotinamide adenine dinucleotide phosphate-driven reactive oxygen species production was also reduced in the aortas of TSA-treated aortic coarctation rats. The vasoconstriction induced by angiotensin II (Ang II, 100 nM) was inhibited by TSA in both endothelium-intact and endothelium-denuded rat aortas, suggesting that TSA has mainly acted in vascular smooth muscle cells (VSMCs). In cultured rat aortic VSMCs, Ang II increased p66shc phosphorylation, which was inhibited by the Ang II receptor type I (AT1R) inhibitor, valsartan (10 microM), but not by the AT2R inhibitor, PD123319. TSA (1~10 microM) inhibited Ang II-induced p66shc phosphorylation in VSMCs and in HEK293T cells expressing AT1R. Taken together, these results suggest that TSA treatment inhibited vasoconstriction and hypertension via inhibition of Ang II-induced phosphorylation of p66shc through AT1R.
MeSH Terms
Figure
Cited by 1 articles
-
α-Isocubebene modulates vascular tone by inhibiting myosin light chain phosphorylation in murine thoracic aorta
Byeong Hyeok Ye, Eun Jung Kim, Seung Eun Baek, Young Whan Choi, So Youn Park, Chi Dae Kim
Korean J Physiol Pharmacol. 2018;22(4):437-445. doi: 10.4196/kjpp.2018.22.4.437.
Reference
-
1. Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997; 89:373–380. PMID: 9150137.
Article2. Usui T, Okada M, Mizuno W, Oda M, Ide N, Morita T, Hara Y, Yamawaki H. HDAC4 mediates development of hypertension via vascular inflammation in spontaneous hypertensive rats. Am J Physiol Heart Circ Physiol. 2012; 302:H1894–H1904. PMID: 22389387.
Article3. Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999; 402:309–313. PMID: 10580504.
Article4. Pellegrini M, Baldari CT. Apoptosis and oxidative stress-related diseases: the p66Shc connection. Curr Mol Med. 2009; 9:392–398. PMID: 19355920.
Article5. Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002; 295:2450–2452. PMID: 11884717.6. Wu Z, Rogers B, Kachi S, Hackett SF, Sick A, Campochiaro PA. Reduction of p66Shc suppresses oxidative damage in retinal pigmented epithelial cells and retina. J Cell Physiol. 2006; 209:996–1005. PMID: 16972253.
Article7. Sun L, Xiao L, Nie J, Liu FY, Ling GH, Zhu XJ, Tang WB, Chen WC, Xia YC, Zhan M, Ma MM, Peng YM, Liu H, Liu YH, Kanwar YS. p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am J Physiol Renal Physiol. 2010; 299:F1014–F1025. PMID: 20739391.
Article8. Kim CS, Jung SB, Naqvi A, Hoffman TA, DeRicco J, Yamamori T, Cole MP, Jeon BH, Irani K. p53 impairs endothelium-dependent vasomotor function through transcriptional upregulation of p66shc. Circ Res. 2008; 103:1441–1450. PMID: 18988897.
Article9. Kang MW, Song HJ, Kang SK, Kim Y, Jung SB, Jee S, Moon JY, Suh KS, Lee SD, Jeon BH, Kim CS. Nafamostat mesilate inhibits TNF-α-Induced vascular endothelial cell dysfunction by inhibiting reactive oxygen species production. Korean J Physiol Pharmacol. 2015; 19:229–234. PMID: 25954127.
Article10. Parker FB Jr, Streeten DH, Farrell B, Blackman MS, Sondheimer HM, Anderson GH Jr. Preoperative and postoperative renin levels in coarctation of the aorta. Circulation. 1982; 66:513–514. PMID: 7046989.
Article11. Lee SK, Kim HS, Song YJ, Joo HK, Lee JY, Lee KH, Cho EJ, Cho CH, Park JB, Jeon BH. Alteration of p66shc is associated with endothelial dysfunction in the abdominal aortic coarctation of rats. FEBS Lett. 2008; 582:2561–2566. PMID: 18588882.
Article12. Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, Irani K, Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 2009; 104:219–227. 5p following 227PMID: 19038866.13. Lee SK, Chung JI, Park MS, Joo HK, Lee EJ, Cho EJ, Park JB, Ryoo S, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease 1 inhibits protein kinase C-mediated p66shc phosphorylation and vasoconstriction. Cardiovasc Res. 2011; 91:502–509. PMID: 21467074.
Article14. Je HD, Kim HD, Jeong JH. The inhibitory effect of eupatilin on the agonist-induced regulation of vascular contractility. Korean J Physiol Pharmacol. 2013; 17:31–36. PMID: 23439928.
Article15. Lee SK, Lee JY, Joo HK, Cho EJ, Kim CS, Lee SD, Park JB, Jeon BH. Tat-mediated p66shc transduction decreased phosphorylation of endothelial nitric oxide synthase in endothelial cells. Korean J Physiol Pharmacol. 2012; 16:199–204. PMID: 22802702.
Article16. Graiani G, Lagrasta C, Migliaccio E, Spillmann F, Meloni M, Madeddu P, Quaini F, Padura IM, Lanfrancone L, Pelicci P, Emanueli C. Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension. 2005; 46:433–440. PMID: 15998704.17. Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006; 5:769–784. PMID: 16955068.
Article18. Brogdon JL, Xu Y, Szabo SJ, An S, Buxton F, Cohen D, Huang Q. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007; 109:1123–1130. PMID: 17008546.
Article19. Adcock IM. HDAC inhibitors as anti-inflammatory agents. Br J Pharmacol. 2007; 150:829–831. PMID: 17325655.
Article20. Davis FJ, Pillai JB, Gupta M, Gupta MP. Concurrent opposite effects of trichostatin A, an inhibitor of histone deacetylases, on expression of alpha-MHC and cardiac tubulins: implication for gain in cardiac muscle contractility. Am J Physiol Heart Circ Physiol. 2005; 288:H1477–H1490. PMID: 15388503.21. Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990; 265:17174–17179. PMID: 2211619.
Article22. Kee HJ, Kwon JS, Shin S, Ahn Y, Jeong MH, Kook H. Trichostatin A prevents neointimal hyperplasia via activation of Krüppel like factor 4. Vascul Pharmacol. 2011; 55:127–134. PMID: 21763782.
Article23. Lee HA, Lee DY, Cho HM, Kim SY, Iwasaki Y, Kim IK. Histone deacetylase inhibition attenuates transcriptional activity of mineralocorticoid receptor through its acetylation and prevents development of hypertension. Circ Res. 2013; 112:1004–1012. PMID: 23421989.24. Salgado HC, Skelton MM, Salgado MC, Cowley AW Jr. Physiopathogenesis of acute aortic coarctation hypertension in conscious rats. Hypertension. 1994; 23(1 Suppl):I78–I81. PMID: 8282379.
Article25. Li W, Liu J, Hammond SL, Tjalkens RB, Saifudeen Z, Feng Y. Angiotensin II regulates brain (pro)renin receptor expression through activation of cAMP response element-binding protein. Am J Physiol Regul Integr Comp Physiol. 2015; DOI: 10.1152/ajpregu.00319.2014.
Article26. Pentz ES, Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol. 2008; 294:H699–H707. PMID: 18055510.
Article27. Song R, Van Buren T, Yosypiv IV. Histone deacetylases are critical regulators of the renin-angiotensin system during ureteric bud branching morphogenesis. Pediatr Res. 2010; 67:573–578. PMID: 20496471.
Article28. Colussi C, Scopece A, Vitale S, Spallotta F, Mattiussi S, Rosati J, Illi B, Mai A, Castellano S, Sbardella G, Farsetti A, Capogrossi MC, Gaetano C. P300/CBP associated factor regulates nitroglycerin-dependent arterial relaxation by N(ε)-lysine acetylation of contractile proteins. Arterioscler Thromb Vasc Biol. 2012; 32:2435–2443. PMID: 22859492.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Angiotensin II on Hypoxic Pulmonary Vasoconstriction in Isolated Rabbit Lung
- Overview of the Renin-Angiotensin System
- p66shc Adaptor Protein Suppresses the Activation of Endothelial Nitric Oxide Synthase in Mouse Embryonic Fibroblasts
- Comparative effects of angiotensin II and angiotensin-(4-8) on blood pressure and ANP secretion in rats
- Renal intercalated cells and blood pressure regulation