Korean J Physiol Pharmacol.
2017 Nov;21(6):667-674. 10.4196/kjpp.2017.21.6.667.
Comparative effects of angiotensin II and angiotensin-(4-8) on blood pressure and ANP secretion in rats
- Affiliations
-
- 1Department of Physiology, Research Institute for Endocrine Sciences, Chonbuk National University Medical School, Jeonju 54907, Korea. shkim@chonbuk.ac.kr
- KMID: 2395262
- DOI: http://doi.org/10.4196/kjpp.2017.21.6.667
Abstract
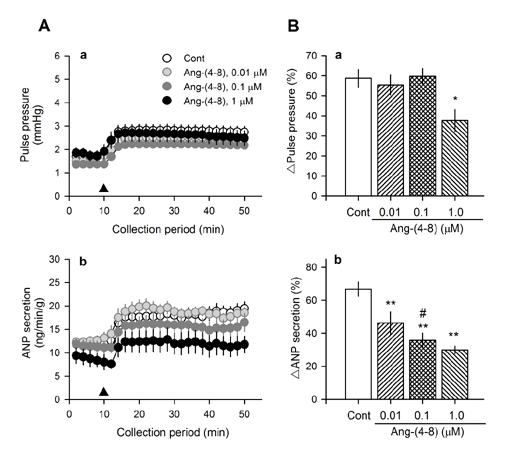
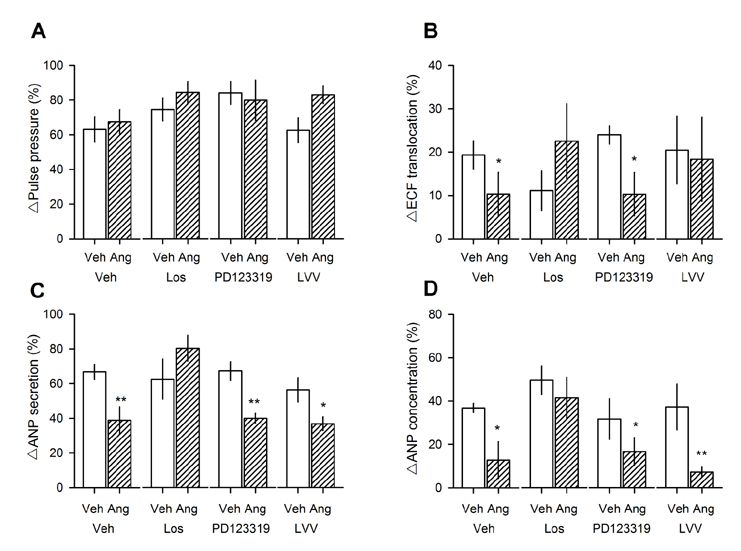
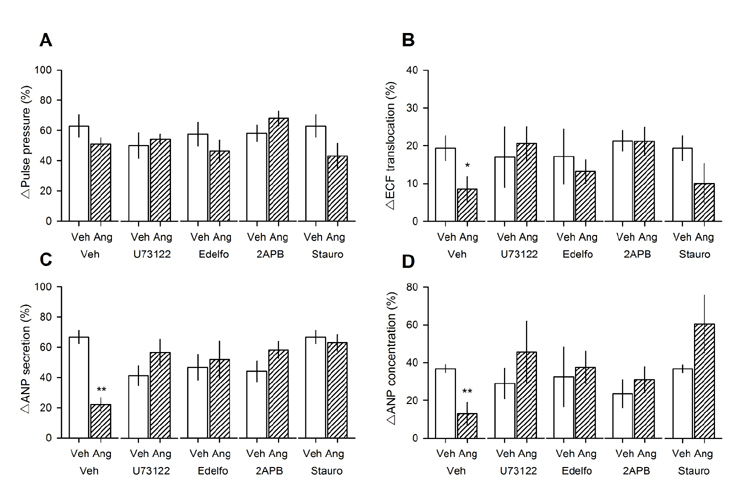
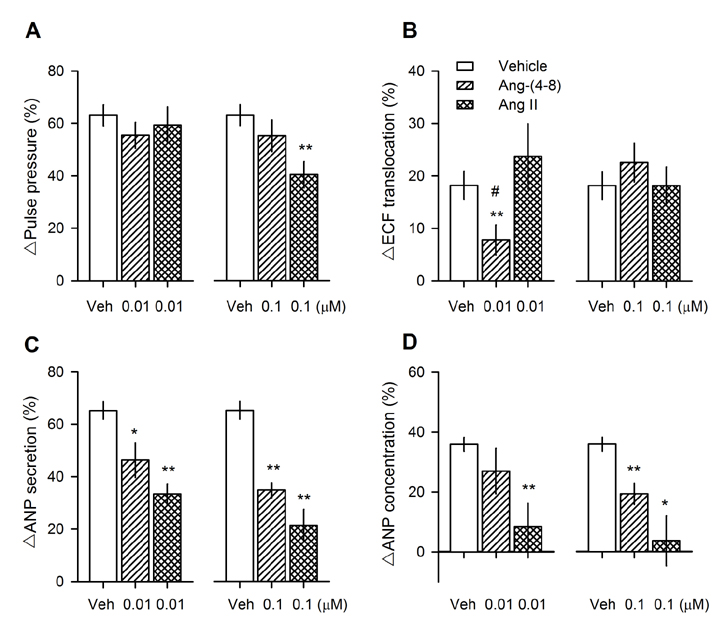
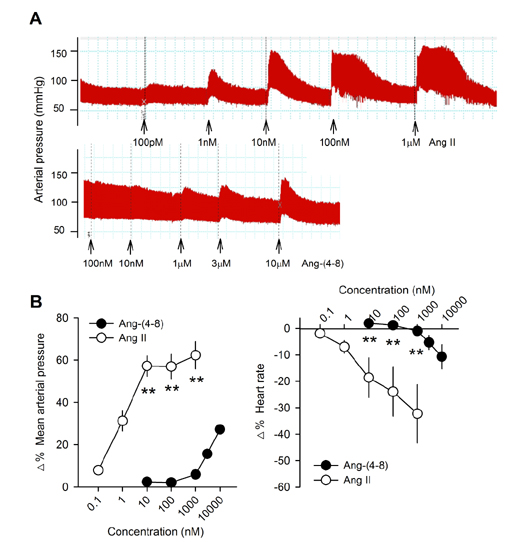
- Angiotensin II (Ang II) is metabolized from N-terminal by aminopeptidases and from C-terminal by Ang converting enzyme (ACE) to generate several truncated angiotensin peptides (Angs). The truncated Angs have different biological effects but it remains unknown whether Ang-(4-8) is an active peptide. The present study was to investigate the effects of Ang-(4-8) on hemodynamics and atrial natriuretic peptide (ANP) secretion using isolated beating rat atria. Atrial stretch caused increases in atrial contractility by 60% and in ANP secretion by 70%. Ang-(4-8) (0.01, 0.1, and 1 µM) suppressed high stretch-induced ANP secretion in a dose-dependent manner. Ang-(4-8) (0.1 µM)-induced suppression of ANP secretion was attenuated by the pretreatment with an antagonist of Ang type 1 receptor (ATâ‚R) but not by an antagonist of ATâ‚‚R or ATâ‚„R. Ang-(4-8)-induced suppression of ANP secretion was attenuated by the pretreatment with inhibitor of phospholipase (PLC), inositol triphosphate (IP₃) receptor, or nonspecific protein kinase C (PKC). The potency of Ang-(4-8) to inhibit ANP secretion was similar to Ang II. However, Ang-(4-8) 10 µM caused an increased mean arterial pressure which was similar to that by 1 nM Ang II. Therefore, we suggest that Ang-(4-8) suppresses high stretch-induced ANP secretion through the ATâ‚R and PLC/IP₃/PKC pathway. Ang-(4-8) is a biologically active peptide which functions as an inhibition mechanism of ANP secretion and an increment of blood pressure.
Keyword
MeSH Terms
-
Aminopeptidases
Angiotensin II*
Angiotensins*
Animals
Arterial Pressure
Atrial Natriuretic Factor*
Blood Pressure*
Heart
Hemodynamics
Inositol
Peptides
Phospholipases
Protein Kinase C
Rats*
Receptor, Angiotensin, Type 1
Signal Transduction
Aminopeptidases
Angiotensin II
Angiotensins
Atrial Natriuretic Factor
Inositol
Peptides
Phospholipases
Protein Kinase C
Receptor, Angiotensin, Type 1
Figure
Reference
-
1. Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003; 24:261–271.2. Guethe LM, Pelegrini-da-Silva A, Borelli KG, Juliano MA, Pelosi GG, Pesquero JB, Silva CL, Corrêa FM, Murad F, Prado WA, Martins AR. Angiotensin (5-8) modulates nociception at the rat periaqueductal gray via the NO-sGC pathway and an endogenous opioid. Neuroscience. 2013; 231:315–327.3. Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008; 264:224–236.4. Semple PF, Boyd AS, Dawes PM, Morton JJ. Angiotensin II and its heptapeptide (2-8), hexapeptide (3-8), and pentapeptide (4-8) metabolites in arterial and venous blood of man. Circ Res. 1976; 39:671–678.5. Wilson WL, Roques BP, Llorens-Cortes C, Speth RC, Harding JW, Wright JW. Roles of brain angiotensins II and III in thirst and sodium appetite. Brain Res. 2005; 1060:108–117.6. Campbell WB, Brooks SN, Pettinger WA. Angiotensin II- and angiotensin 3-induced aldosterone release vivo in the rat. Science. 1974; 184:994–996.7. Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, Sanada H. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology. 2011; 152:1582–1588.8. Zini S, Fournie-Zaluski MC, Chauvel E, Roques BP, Corvol P, Llorens-Cortes C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc Natl Acad Sci U S A. 1996; 93:11968–11973.9. Bader M. ACE2, angiotensin-(1–7), and Mas: the other side of the coin. Pflugers Arch. 2013; 465:79–85.10. Kono T, Oseko F, Ikeda F, Nakano R, Taniguchi A, Imura H, Khosla MC. Biological activity of des-(Asp1, Arg2, Val3)-angiotensin II in man. Life Sci. 1983; 32:337–343.11. McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005; 16:469–477.12. Dietz JR. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc Res. 2005; 68:8–17.13. Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006; 27:47–72.14. Oh YB, Gao S, Shah A, Kim JH, Park WH, Kim SH. Endogenous angiotensin II suppresses stretch-induced ANP secretion via AT1 receptor pathway. Peptides. 2011; 32:374–381.15. Oh YB, Gao S, Lim JM, Kim HT, Park BH, Kim SH. Caveolae are essential for angiotensin II type 1 receptor-mediated ANP secretion. Peptides. 2011; 32:1422–1430.16. Park BM, Oh YB, Gao S, Cha SA, Kang KP, Kim SH. Angiotensin III stimulates high stretch-induced ANP secretion via angiotensin type 2 receptor. Peptides. 2013; 42:131–137.17. Park BM, Gao S, Cha SA, Park BH, Kim SH. Cardioprotective effects of angiotensin III against ischemic injury via the AT2 receptor and KATP channels. Physiol Rep. 2013; 1:e00151.18. Park BM, Cha SA, Lee SH, Kim SH. Angiotensin IV protects cardiac reperfusion injury by inhibiting apoptosis and inflammation via AT4R in rats. Peptides. 2016; 79:66–74.19. Cha SA, Park BM, Gao S, Kim SH. Stimulation of ANP by angiotensin-(1-9) via the angiotensin type 2 receptor. Life Sci. 2013; 93:934–940.20. Yu L, Yuan K, Phuong HT, Park BM, Kim SH. Angiotensin-(1-5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides. 2016; 86:33–41.21. Shah A, Oh YB, Shan G, Song CH, Park BH, Kim SH. Angiotensin-(1-7) attenuates hyposmolarity-induced ANP secretion via the Na+-K+ pump. Peptides. 2010; 31:1779–1785.22. Shah A, Gul R, Yuan K, Gao S, Oh YB, Kim UH, Kim SH. Angiotensin-(1-7) stimulates high atrial pacing-induced ANP secretion via Mas/PI3-kinase/Akt axis and Na+/H+ exchanger. Am J Physiol Heart Circ Physiol. 2010; 298:H1365–H1374.23. Yuan K, Cao C, Han JH, Kim SZ, Kim SH. Adenosine-stimulated atrial natriuretic peptide release through A1 receptor subtype. Hypertension. 2005; 46:1381–1387.24. Han JH, Bai GY, Park JH, Yuan K, Park WH, Kim SZ, Kim SH. Regulation of stretch-activated ANP secretion by chloride channels. Peptides. 2008; 29:613–621.25. Cui X, Wen JF, Jin JY, Xu WX, Kim SZ, Kim SH, Lee HS, Cho KW. Protein kinase-dependent and Ca2+-independent cAMP inhibition of ANP release in beating rabbit atria. Am J Physiol Regul Integr Comp Physiol. 2002; 282:R1477–R1489.26. Cho KW, Seul KH, Ryu H, Kim SH, Koh GY. Characteristics of distension-induced release of immunoreactive atrial natriuretic peptide in isolated perfused rabbit atria. Regul Pept. 1988; 22:333–345.27. Cho KW, Lee SJ, Wen JF, Kim SH, Seul KH, Lee HS. Mechanical control of extracellular space in rabbit atria: an intimate modulator of the translocation of extracellular fluid and released atrial natriuretic peptide. Exp Physiol. 2002; 87:185–194.28. Cho KW, Kim SH, Hwang YH, Seul KH. Extracellular fluid translocation in perfused rabbit atria: implication in control of atrial natriuretic peptide secretion. J Physiol. 1993; 468:591–607.29. Braszko JJ, WŁasienko J, Kupryszewski G, Witczuk B, Wisniewski K. Behavioral effects of angiotensin II and angiotensin II-(4-8)-pentapeptide in rats. Physiol Behav. 1988; 44:327–332.30. Ptasinska-Wnuk D, Mucha SA, Lawnicka H, Fryczak J, Kunert-Radek J, Pawlikowski M, Stepien H. The effects of angiotensin peptides and angiotensin receptor antagonists on the cell growth and angiogenic activity of GH3 lactosomatotroph cells in vitro. Endocrine. 2012; 42:88–96.31. Vivar R, Soto C, Copaja M, Mateluna F, Aranguiz P, Muñoz JP, Chiong M, Garcia L, Letelier A, Thomas WG, Lavandero S, Díaz-Araya G. Phospholipase C/protein kinase C pathway mediates angiotensin II-dependent apoptosis in neonatal rat cardiac fibroblasts expressing AT1 receptor. J Cardiovasc Pharmacol. 2008; 52:184–190.32. Ramracheya RD, Muller DS, Wu Y, Whitehouse BJ, Huang GC, Amiel SA, Karalliedde J, Viberti G, Jones PM, Persaud SJ. Direct regulation of insulin secretion by angiotensin II in human islets of Langerhans. Diabetologia. 2006; 49:321–331.33. Kono T, Taniguchi A, Imura H, Oseko F, Khosla MC. Biological activities of angiotensin II-(1-6)-hexapeptide and angiotensin II-(1-7)-heptapeptide in man. Life Sci. 1986; 38:1515–1519.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renin Angiotensin System in Rabbit Corpus Cavernosum: Functional Characterization of Angiotensin II Receptors
- Effects of intracerebroventricular angiotensin II on the cardiovasc- ular and endocrine systems in conscius normotensive and hypertensi- ve rats
- Effects of intracerebroventricular angiotensin II on the response to hemorrhage in conscious normotensive and hypertensive rats
- An experimental study on radioprotective effects of angiotensin II on gastrointestinal tract
- Overview of the Renin-Angiotensin System