Korean J Physiol Pharmacol.
2014 Dec;18(6):497-502. 10.4196/kjpp.2014.18.6.497.
Scant Extracellular NAD Cleaving Activity of Human Neutrophils is Down-Regulated by fMLP via FPRL1
- Affiliations
-
- 1Department of Pharmacology, Infectious Diseases Medical Research Center, College of Medicine, Hallym University, Chuncheon 200-702, Korea. dksong@hallym.ac.kr
- KMID: 2285552
- DOI: http://doi.org/10.4196/kjpp.2014.18.6.497
Abstract
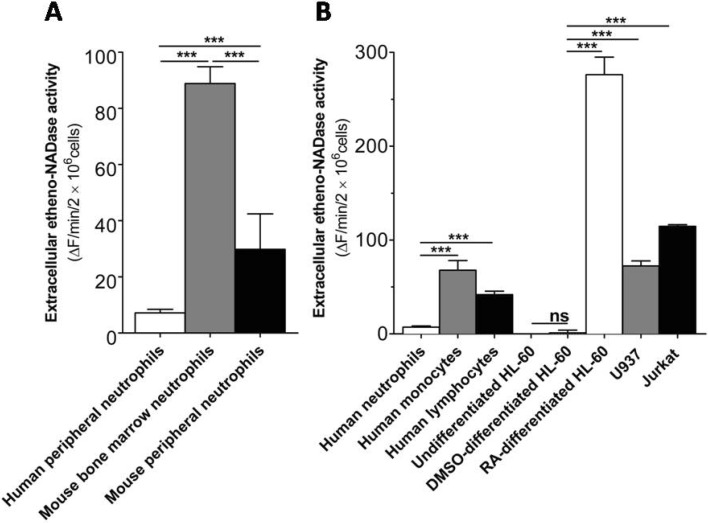
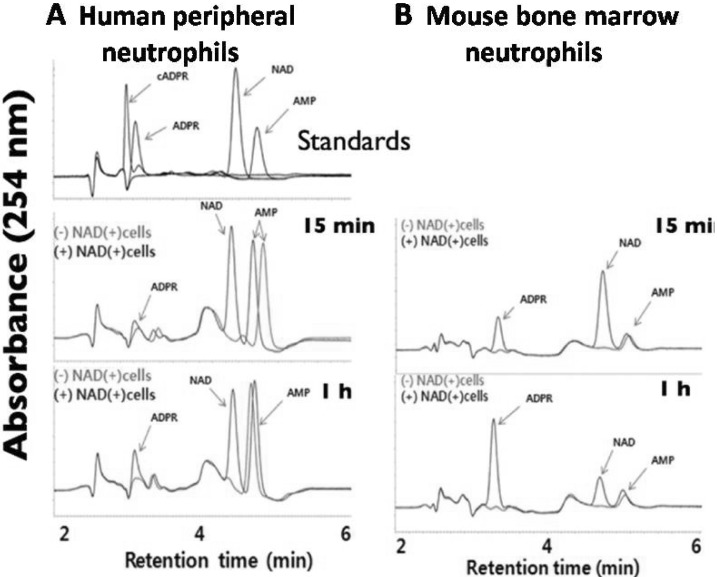
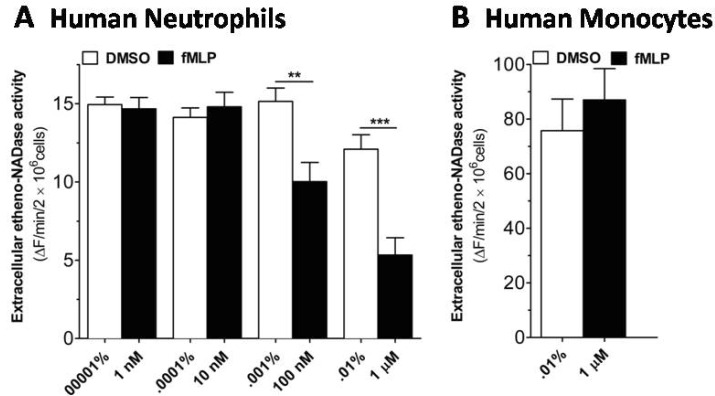
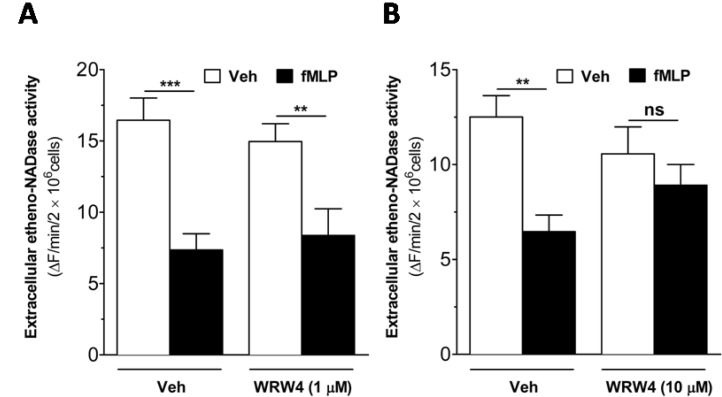
- Extracellular nicotinamide adenine dinucleotide (NAD) cleaving activity of a particular cell type determines the rate of the degradation of extracellular NAD with formation of metabolites in the vicinity of the plasma membrane, which has important physiological consequences. It is yet to be elucidated whether intact human neutrophils have any extracellular NAD cleaving activity. In this study, with a simple fluorometric assay utilizing 1,N6-ethenoadenine dinucleotide (etheno-NAD) as the substrate, we have shown that intact peripheral human neutrophils have scant extracellular etheno-NAD cleaving activity, which is much less than that of mouse bone marrow neutrophils, mouse peripheral neutrophils, human monocytes and lymphocytes. With high performance liquid chromatography (HPLC), we have identified that ADP-ribose (ADPR) is the major extracellular metabolite of NAD degradation by intact human neutrophils. The scant extracellular etheno-NAD cleaving activity is decreased further by N-formyl-methionine-leucine-phenylalanine (fMLP), a chemoattractant for neutrophils. The fMLP-mediated decrease in the extracellular etheno-NAD cleaving activity is reversed by WRW4, a potent FPRL1 antagonist. These findings show that a much less extracellular etheno-NAD cleaving activity of intact human neutrophils compared to other immune cell types is down-regulated by fMLP via a low affinity fMLP receptor FPRL1.
MeSH Terms
Figure
Reference
-
1. Grahnert A, Grahnert A, Klein C, Schilling E, Wehrhahn J, Hauschildt S. Review: NAD+: a modulator of immune functions. Innate Immun. 2011; 17:212–233. PMID: 20388721.2. Muller HM, Muller CD, Schuber F. NAD+ glycohydrolase, an ecto-enzyme of calf spleen cells. Biochem J. 1983; 212:459–464. PMID: 6192807.
Article3. Pekala PH, Anderson BM. Studies of bovine erythrocyte NAD glycohydrolase. J Biol Chem. 1978; 253:7453–7459. PMID: 212424.
Article4. Balducci E, Micossi LG. NAD-dependent inhibition of the NADglycohydrolase activity in A549 cells. Mol Cell Biochem. 2002; 233:127–132. PMID: 12083366.5. Wang J, Nemoto E, Kots AY, Kaslow HR, Dennert G. Regulation of cytotoxic T cells by ecto-nicotinamide adenine dinucleotide (NAD) correlates with cell surface GPI-anchored/arginine ADP-ribosyltransferase. J Immunol. 1994; 153:4048–4058. PMID: 7930612.6. Okazaki IJ, Zolkiewska A, Takada T, Moss J. Characterization of mammalian ADP-ribosylation cycles. Biochimie. 1995; 77:319–325. PMID: 8527484.
Article7. Bortell R, Moss J, McKenna RC, Rigby MR, Niedzwiecki D, Stevens LA, Patton WA, Mordes JP, Greiner DL, Rossini AA. Nicotinamide adenine dinucleotide (NAD) and its metabolites inhibit T lymphocyte proliferation: role of cell surface NAD glycohydrolase and pyrophosphatase activities. J Immunol. 2001; 167:2049–2059. PMID: 11489987.
Article8. Bruzzone S, Moreschi I, Guida L, Usai C, Zocchi E, De Flora A. Extracellular NAD+ regulates intracellular calcium levels and induces activation of human granulocytes. Biochem J. 2006; 393:697–704. PMID: 16225456.
Article9. Gerth A, Nieber K, Oppenheimer NJ, Hauschildt S. Extracellular NAD+ regulates intracellular free calcium concentration in human monocytes. Biochem J. 2004; 382:849–856. PMID: 15233622.
Article10. Podestà M, Zocchi E, Pitto A, Usai C, Franco L, Bruzzone S, Guida L, Bacigalupo A, Scadden DT, Walseth TF, De Flora A, Daga A. Extracellular cyclic ADP-ribose increases intracellular free calcium concentration and stimulates proliferation of human hemopoietic progenitors. FASEB J. 2000; 14:680–690. PMID: 10744625.
Article11. Repnik U, Knezevic M, Jeras M. Simple and cost-effective isolation of monocytes from buffy coats. J Immunol Methods. 2003; 278:283–292. PMID: 12957415.
Article12. Porfiri E, Hoffbrand AV, Wickremasinghe RG. Retinoic acidinduced granulocytic differentiation of HL60 human promyelocytic leukemia cells is preceded by downregulation of autonomous generation of inositol lipid-derived second messengers. Blood. 1991; 78:1069–1077. PMID: 1651133.
Article13. Funaro A, Ortolan E, Ferranti B, Gargiulo L, Notaro R, Luzzatto L, Malavasi F. CD157 is an important mediator of neutrophil adhesion and migration. Blood. 2004; 104:4269–4278. PMID: 15328157.
Article14. Kontani K, Nishina H, Ohoka Y, Takahashi K, Katada T. NAD glycohydrolase specifically induced by retinoic acid in human leukemic HL-60 cells. Identification of the NAD glycohydrolase as leukocyte cell surface antigen CD38. J Biol Chem. 1993; 268:16895–16898. PMID: 8394323.
Article15. Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002; 23:541–548. PMID: 12401407.
Article16. Jung YY, Nam Y, Park YS, Lee HS, Hong SA, Kim BK, Park ES, Chung YH, Jeong JH. Protective effect of phosphatidylcholine on lipopolysaccharide-induced acute inflammation in multiple organ injury. Korean J Physiol Pharmacol. 2013; 17:209–216. PMID: 23776397.
Article17. Haag F, Adriouch S, Braβ A, Jung C, Möller S, Scheuplein F, Bannas P, Seman M, Koch-Nolte F. Extracellular NAD and ATP: Partners in immune cell modulation. Purinergic Signal. 2007; 3:71–81. PMID: 18404420.
Article18. Pfister M, Ogilvie A, da Silva CP, Grahnert A, Guse AH, Hauschildt S. NAD degradation and regulation of CD38 expression by human monocytes/macrophages. Eur J Biochem. 2001; 268:5601–5608. PMID: 11683883.
Article19. Takasawa S, Tohgo A, Noguchi N, Koguma T, Nata K, Sugimoto T, Yonekura H, Okamoto H. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J Biol Chem. 1993; 268:26052–26054. PMID: 8253715.
Article20. Zocchi E, Franco L, Guida L, Benatti U, Bargellesi A, Malavasi F, Lee HC, De Flora A. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993; 196:1459–1465. PMID: 8250903.
Article21. Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993; 262:1056–1059. PMID: 8235624.
Article22. Berthelier V, Tixier JM, Muller-Steffner H, Schuber F, Deterre P. Human CD38 is an authentic NAD(P)+ glycohydrolase. Biochem J. 1998; 330:1383–1390. PMID: 9494110.
Article23. Yamamoto-Katayama S, Ariyoshi M, Ishihara K, Hirano T, Jingami H, Morikawa K. Crystallographic studies on human BST-1/CD157 with ADP-ribosyl cyclase and NAD glycohydrolase activities. J Mol Biol. 2002; 316:711–723. PMID: 11866528.
Article24. Ortolan E, Vacca P, Capobianco A, Armando E, Crivellin F, Horenstein A, Malavasi F. CD157, the Janus of CD38 but with a unique personality. Cell Biochem Funct. 2002; 20:309–322. PMID: 12415565.
Article25. Ortolan E, Tibaldi EV, Ferranti B, Lavagno L, Garbarino G, Notaro R, Luzzatto L, Malavasi F, Funaro A. CD157 plays a pivotal role in neutrophil transendothelial migration. Blood. 2006; 108:4214–4222. PMID: 16917007.
Article26. Fujita T, Zawawi KH, Kurihara H, Van Dyke TE. CD38 cleavage in fMLP- and IL-8-induced chemotaxis is dependent on p38 MAP kinase but independent of p44/42 MAP kinase. Cell Signal. 2005; 17:167–175. PMID: 15494208.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracellular Mechanisms of Neutrophils in Immune Cell Crosstalk
- Interleukin-8 Expression by Human Neutrophils Activated by Water Soluble Proteins of Helicobacter pylori
- Lysophosphatidylglycerol inhibits formyl peptide receptor like-1-stimulated chemotactic migration and IL-1beta production from human phagocytes
- Superoxide Production and Myeloperoxidase Activity by Peripheral Neutrophils in the Bronchial Asthmatics
- LL-37 inhibits serum amyloid A-induced IL-8 production in human neutrophils