Effect of Berberine on Depression- and Anxiety-Like Behaviors and Activation of the Noradrenergic System Induced by Development of Morphine Dependence in Rats
- Affiliations
-
- 1Acupuncture and Meridian Science Research Center, College of Oriental Medicine, Kyung Hee University, Seoul 130-701, Korea. bombi@khu.ac.kr
- 2The Graduate School of Basic Science of Oriental Medicine, College of Oriental Medicine, Kyung Hee University, Seoul 130-701, Korea.
- KMID: 2285445
- DOI: http://doi.org/10.4196/kjpp.2012.16.6.379
Abstract
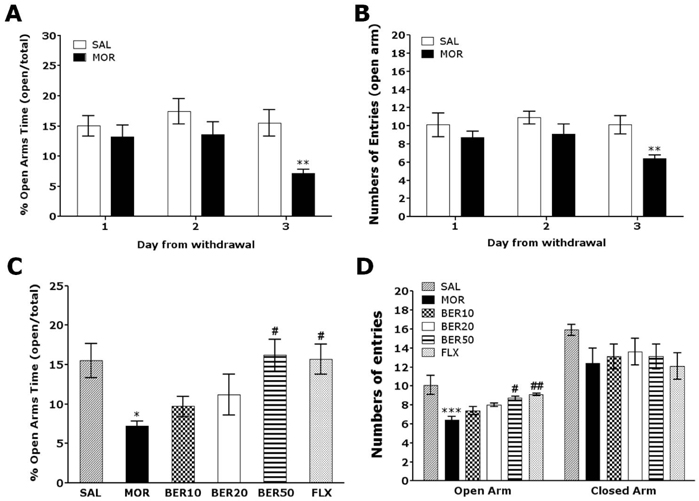
- The purpose of this study was to evaluate whether berberine (BER) administration could attenuate depression- and anxiety-like behaviors and increase corticotrophin-releasing factor (CRF) and tyrosine hydroxylase (TH) expression following chronic morphine withdrawal in rats. Male rats were exposed to chronic, intermittent, escalating morphine (10~50 mg/kg) for 10 days. After the last morphine injection, depression- and anxiety-like beahvior associated with morphine discontinuation persisted for at least three days during withdrawal without any change in ambulatory activity. Daily BER administration significantly decreased immobility in the forced swimming test and increased open-arm exploration in the elevated plus maze test. BER administration also significantly blocked the increase in hypothalamic CRF expression and TH expression in the locus coeruleus (LC) and the decrease in hippocampal brain-derived neurotrophic factor (BDNF) mRNA expression. Taken together, these findings demonstrated that BER administration significantly reduced morphine withdrawal-associated behaviors following discontinuation of repeated morphine administration in rats, possibly through modulation of hypothalamic CRF and the central noradrenergic system. BER may be a useful agent for treating or alleviating complex withdrawal symptoms and preventing morphine use relapses.
MeSH Terms
Figure
Cited by 3 articles
-
Berberine alleviates symptoms of anxiety by enhancing dopamine expression in rats with post-traumatic stress disorder
Bombi Lee, Insop Shim, Hyejung Lee, Dae-Hyun Hahm
Korean J Physiol Pharmacol. 2018;22(2):183-192. doi: 10.4196/kjpp.2018.22.2.183.Ineffective Doses of Dexmedetomidine Potentiates the Antinociception Induced by Morphine and Fentanyl in Acute Pain Model
Mumin Unal, Sinan Gursoy, Ahmet Altun, Cevdet Duger, Iclal Ozdemir Kol, Kenan Kaygusuz, Ihsan Bagcivan, Caner Mimaroglu
Korean J Physiol Pharmacol. 2013;17(5):417-422. doi: 10.4196/kjpp.2013.17.5.417.Atypical Antidepressant Activity of 3,4-Bis(3,4-Dimethoxyphenyl) Furan-2,5-Dione Isolated from Heart Wood of
Cedrus deodara , in Rodents
Nitesh Kumar, Daniel Dhayabaran, Madhavan Nampoothiri, Krishnadas Nandakumar, A. Puratchikody, Natasha Lalani, Karima Dawood, Aanesha Ghosh
Korean J Physiol Pharmacol. 2014;18(5):365-369. doi: 10.4196/kjpp.2014.18.5.365.
Reference
-
1. Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, Kalivas PW. Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology (Berl). 2009. 203:677–684.2. Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011. 69:236–244.3. Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berl). 2001. 157:217–220.4. Maj M, Turchan J, Smiałowska M, Przewłocka B. Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides. 2003. 37:105–110.5. Lee B, Kim H, Shim I, Lee H, Hahm DH. Wild ginseng attenuates anxiety- and depression-like behaviors during morphine withdrawal. J Microbiol Biotechnol. 2011. 21:1088–1096.6. Mochizuki D, Tsujita R, Yamada S, Kawasaki K, Otsuka Y, Hashimoto S, Hattori T, Kitamura Y, Miki N. Neurochemical and behavioural characterization of milnacipran, a serotonin and noradrenaline reuptake inhibitor in rats. Psychopharmacology (Berl). 2002. 162:323–332.7. Zhao Z, Zhang HT, Bootzin E, Millan MJ, O'Donnell JM. Association of changes in norepinephrine and serotonin transporter expression with the long-term behavioral effects of antidepressant drugs. Neuropsychopharmacology. 2009. 34:1467–1481.8. Cui HS, Matsumoto K, Murakami Y, Hori H, Zhao Q, Obi R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures: involvement of B-cell lymphoma 2 phosphorylation suppression. Biol Pharm Bull. 2009. 32:79–85.9. Zhang J, Yang JQ, He BC, Zhou QX, Yu HR, Tang Y, Liu BZ. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi Med J. 2009. 30:760–766.10. Peng WH, Lo KL, Lee YH, Hung TH, Lin YC. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 2007. 81:933–938.11. Peng WH, Wu CR, Chen CS, Chen CF, Leu ZC, Hsieh MT. Anxiolytic effect of berberine on exploratory activity of the mouse in two experimental anxiety models: interaction with drugs acting at 5-HT receptors. Life Sci. 2004. 75:2451–2462.12. Erdtmann-Vourliotis M, Mayer P, Linke R, Riechert U, Höllt V. Long-lasting sensitization towards morphine in motoric and limbic areas as determined by c-fos expression in rat brain. Brain Res Mol Brain Res. 1999. 72:1–16.13. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1986. New York: Academic Press.14. Vieira C, De Lima TC, Carobrez Ade P, Lino-de-Oliveira C. Frequency of climbing behavior as a predictor of altered motor activity in rat forced swimming test. Neurosci Lett. 2008. 445:170–173.15. Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, Umathe S, Mundhada D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011. 220:30–41.16. Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol. 2008. 589:163–172.17. Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 2006. 7:78.18. Eaker EY, Sninsky CA. Effect of berberine on myoelectric activity and transit of the small intestine in rats. Gastroenterology. 1989. 96:1506–1513.19. Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005. 4:775–790.20. Hosseinmardi N, Fathollahi Y, Naghdi N, Javan M. Theta pulse stimulation: a natural stimulus pattern can trigger long-term depression but fails to reverse long-term potentiation in morphine withdrawn hippocampus area CA1. Brain Res. 2009. 1296:1–14.21. Consoni FT, Vital MA, Andreatini R. Dual monoamine modulation for the antidepressant-like effect of lamotrigine in the modified forced swimming test. Eur Neuropsychopharmacol. 2006. 16:451–458.22. Garcia-Carmona JA, Almela P, Baroja-Mazo A, Milanes MV, Laorden ML. Restricted role of CRF1 receptor for the activity of brainstem catecholaminergic neurons in the negative state of morphine withdrawal. Psychopharmacology (Berl). 2012. 220:379–393.23. Núñez C, Földes A, Pérez-Flores D, García-Borrín JC, Laorden ML, Kovács KJ, Milanés MV. Elevated glucocorticoid levels are responsible for induction of tyrosine hydroxylase mRNA expression, phosphorylation, and enzyme activity in the nucleus of the solitary tract during morphine withdrawal. Endocrinology. 2009. 150:3118–3127.24. Redmond DE Jr, Huang YH. The primate locus coeruleus and effects of clonidine on opiate withdrawal. J Clin Psychiatry. 1982. 43:25–29.25. Melia KR, Rasmussen K, Terwilliger RZ, Haycock JW, Nestler EJ, Duman RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992. 58:494–502.26. Park HJ, Shim HS, Kim H, Kim KS, Lee H, Hahm DH, Shim I. Effects of glycyrrhizae radix on repeated restraint stress-induced neurochemical and behavioral responses. Korean J Physiol Pharmacol. 2010. 14:371–376.27. Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y. Anxiety profile in morphine-dependent and withdrawn rats: effect of voluntary exercise. Physiol Behav. 2012. 105:195–202.28. Rezayof A, Hosseini SS, Zarrindast MR. Effects of morphine on rat behaviour in the elevated plus maze: the role of central amygdala dopamine receptors. Behav Brain Res. 2009. 202:171–178.29. Aguiar DC, Terzian AL, Guimarães FS, Moreira FA. Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology (Berl). 2009. 205:217–225.30. Shi J, Li SX, Zhang XL, Wang X, Le Foll B, Zhang XY, Kosten TR, Lu L. Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am J Drug Alcohol Abuse. 2009. 35:267–272.31. Navarro-Zaragoza J, Núñez C, Laorden ML, Milanés MV. Effects of corticotropin-releasing factor receptor-1 antagonists on the brain stress system responses to morphine withdrawal. Mol Pharmacol. 2010. 77:864–873.32. Papaleo F, Kitchener P, Contarino A. Disruption of the CRF/CRF1 receptor stress system exacerbates the somatic signs of opiate withdrawal. Neuron. 2007. 53:577–589.33. Núñez C, Féldes A, Pérez-Flores D, García-Borrín JC, Laorden ML, Kovács KJ, Milanés MV. Elevated glucocorticoid levels are responsible for induction of tyrosine hydroxylase mRNA expression, phosphorylation, and enzyme activity in the nucleus of the solitary tract during morphine withdrawal. Endocrinology. 2009. 150:3118–3127.34. McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci. 2005. 25:6005–6015.35. Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology (Berl). 2003. 170:80–88.36. Cameron OG. Anxious-depressive comorbidity: effects on HPA axis and CNS noradrenergic functions. Essent Psychopharmacol. 2006. 7:24–34.37. Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003. 23:742–747.38. Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004. 5:69–83.39. Chu NN, Zuo YF, Meng L, Lee DY, Han JS, Cui CL. Peripheral electrical stimulation reversed the cell size reduction and increased BDNF level in the ventral tegmental area in chronic morphine-treated rats. Brain Res. 2007. 1182:90–98.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distinct Effects of Non-absorbed Agents Rifaximin and Berberine on the Microbiota-Gut-Brain Axis in Dysbiosis-induced Visceral Hypersensitivity in Rats
- Chronic Administration of Catechin Decreases Depression and Anxiety-Like Behaviors in a Rat Model Using Chronic Corticosterone Injections
- Intrathecal Lamotrigine Attenuates Antinociceptive Morphine Tolerance and Suppresses Spinal Glial Cell Activation in Morphine-Tolerant Rats
- Berberine alleviates symptoms of anxiety by enhancing dopamine expression in rats with post-traumatic stress disorder
- Limonene Inhibits Methamphetamine-Induced Sensitizations via the Regulation of Dopamine Receptor Supersensitivity