J Korean Med Sci.
2013 Feb;28(2):300-307. 10.3346/jkms.2013.28.2.300.
Intrathecal Lamotrigine Attenuates Antinociceptive Morphine Tolerance and Suppresses Spinal Glial Cell Activation in Morphine-Tolerant Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jongyeon_park@amc.seoul.kr
- KMID: 1429208
- DOI: http://doi.org/10.3346/jkms.2013.28.2.300
Abstract
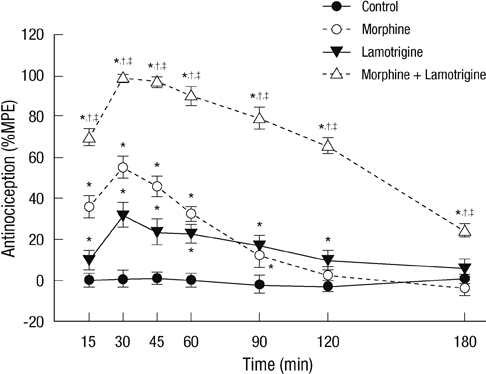
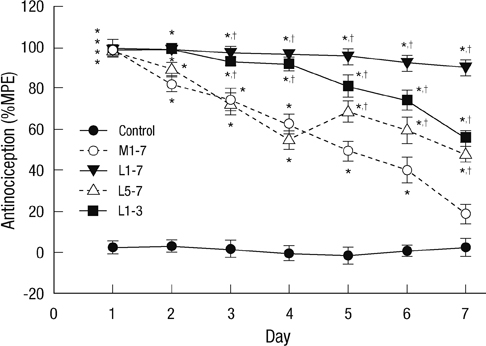
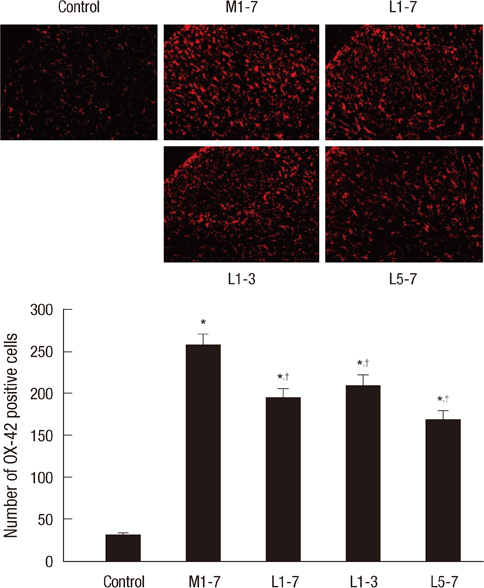
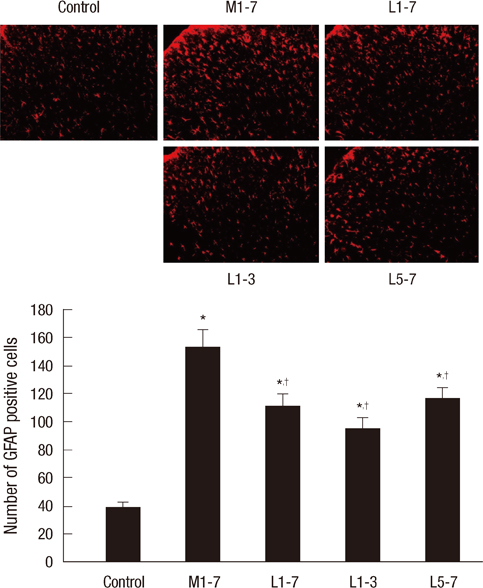
- Glial cells play a critical role in morphine tolerance, resulting from repeated administration of morphine. Both the development and the expression of tolerance are suppressed by the analgesic lamotrigine. This study investigated the relationship between the ability of lamotrigine to maintain the antinociceptive effect of morphine during tolerance development and glial cell activation in the spinal cord. In a rat model, morphine (15 microg) was intrathecally injected once daily for 7 days to induce morphine tolerance. Lamotrigine (200 microg) was co-administered with morphine either for 7 days or the first or last 3 days of this 7 day period. Thermal nociception was measured. OX-42 and GFAP immunoreactivity, indicating spinal microglial and astrocytic activation were evaluated on day 8. Tolerance developed after 7 days of intrathecal morphine administration; however, this was completely blocked and reversed by co-administration of lamotrigine. When lamotrigine was coinjected with morphine on days 5-7, the morphine effect was partially restored. Glial cell activation increased with the development of morphine tolerance but was clearly inhibited in the presence of lamotrigine. These results suggest that, in association with the suppression of spinal glial cell activity, intrathecally coadministered lamotrigine attenuates antinociceptive tolerance to morphine.
Keyword
MeSH Terms
-
Analgesics/*pharmacology
Animals
Antigens, CD11b/metabolism
Astrocytes/cytology
Drug Tolerance
Immunohistochemistry
Male
Microglia/cytology
Morphine/*pharmacology
Nerve Tissue Proteins/metabolism
Neuroglia/cytology/*metabolism
Rats
Rats, Sprague-Dawley
Spinal Cord/*cytology
Triazines/*pharmacology
Analgesics
Antigens, CD11b
Nerve Tissue Proteins
Triazines
Morphine
Figure
Reference
-
1. Starowicz K, Sieja A, Bilecki W, Obara I, Przewlocka B. The effect of morphine on MC4 and CRF receptor mRNAs in the rat amygdala and attenuation of tolerance after their blockade. Brain Res. 2003. 990:113–119.2. Cortinez LI, Brandes V, Muñoz HR, Munoz HR, Guerrero ME, Mur M. No clinical evidence of acute opioid tolerance after remifentanil-based anaesthesia. Br J Anaesth. 2001. 87:866–869.3. Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999. 96:7731–7736.4. Kest B, McLemore G, Kao B, Inturrisi CE. The competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist LY293558 attenuates and reverses analgesic tolerance to morphine but not to delta or kappa opioids. J Pharmacol Exp Ther. 1997. 283:1249–1255.5. Vanderah TW, Ossipov MH, Lai J, Malan TP Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001. 92:5–9.6. Powell KJ, Ma W, Sutak M, Doods H, Quirion R, Jhamandas K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br J Pharmacol. 2000. 131:875–884.7. Powell KJ, Hosokawa A, Bell A, Sutak M, Milne B, Quirion R, Jhamandas K. Comparative effects of cyclo-oxygenase and nitric oxide synthase inhibition on the development and reversal of spinal opioid tolerance. Br J Pharmacol. 1999. 127:631–644.8. Lin SL, Tsai RY, Shen CH, Lin FH, Wang JJ, Hsin ST, Wong CS. Co-administration of ultra-low dose naloxone attenuates morphine tolerance in rats via attenuation of NMDA receptor neurotransmission and suppression of neuroinflammation in the spinal cords. Pharmacol Biochem Behav. 2010. 96:236–245.9. Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002. 22:9980–9989.10. Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009. 29:998–1005.11. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005. 6:521–532.12. Chavooshi B, Saberi M, Pournaghash Tehrani S, Bakhtiarian A, Ahmadiani A, Haghparast A. Vigabatrin attenuates the development and expression of tolerance to morphine-induced antinociception in mice. Pharmacol Biochem Behav. 2009. 93:155–159.13. Lees G, Leach MJ. Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamictal) using primary neurological cultures from rat cortex. Brain Res. 1993. 612:190–199.14. Klamt JG. Effects of intrathecally administered lamotrigine, a glutamate release inhibitor, on short- and long-term models of hyperalgesia in rats. Anesthesiology. 1998. 88:487–494.15. Habibi-Asl B, Hassanzadeh K, Vafai H, Mohammadi S. Development of morphine induced tolerance and withdrawal symptoms is attenuated by lamotrigine and magnesium sulfate in mice. Pak J Biol Sci. 2009. 12:798–803.16. Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005. 10:808–819.17. Perisic T, Zimmermann N, Kirmeier T, Asmus M, Tuorto F, Uhr M, Holsboer F, Rein T, Zschocke J. Valproate and amitriptyline exert common and divergent influences on global and gene promoter-specific chromatin modifications in rat primary astrocytes. Neuropsychopharmacology. 2010. 35:792–805.18. Kim DS, Kim JE, Kwak SE, Choi HC, Song HK, Kimg YI, Choi SY, Kang TC. Up-regulated astroglial TWIK-related acid-sensitive K+ channel-1 (TASK-1) in the hippocampus of seizure-sensitive gerbils: a target of anti-epileptic drugs. Brain Res. 2007. 1185:346–358.19. Pavone A, Cardile V. An in vitro study of new antiepileptic drugs and astrocytes. Epilepsia. 2003. 44:34–39.20. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976. 17:1031–1036.21. Jun IG, Park JY, Choi YS, Kim TH. Intrathecal lamotrigine blocks and reverses antinociceptive morphine tolerance in rats. Korean J Anesthesiol. 2009. 56:687–692.22. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988. 32:77–88.23. Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008. 22:114–123.24. Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995. 62:259–274.25. Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006. 124:77–86.26. Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, Tsai SK, Chang YC, Wong CS. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem. 2005. 94:742–752.27. Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995. 61:353–364.28. Elliott K, Kest B, Man A, Kao B, Inturrisi CE. N-methyl-D-aspartate (NMDA) receptors, mu and kappa opioid tolerance, and perspectives on new analgesic drug development. Neuropsychopharmacology. 1995. 13:347–356.29. Wong CS, Hsu MM, Chou R, Chou YY, Tung CS. Intrathecal cyclooxygenase inhibitor administration attenuates morphine antinociceptive tolerance in rats. Br J Anaesth. 2000. 85:747–751.30. Wen ZH, Chang YC, Cherng CH, Wang JJ, Tao PL, Wong CS. Increasing of intrathecal CSF excitatory amino acids concentration following morphine challenge in morphine-tolerant rats. Brain Res. 2004. 995:253–259.31. Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004. 24:7353–7365.32. Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003. 521:1–21.33. Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001. 39:281–286.34. Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000. 20:251–258.35. Rasley A, Bost KL, Olson JK, Miller SD, Marriott I. Expression of functional NK-1 receptors in murine microglia. Glia. 2002. 37:258–267.36. De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006. 122:17–21.37. Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002. 87:721–731.38. Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007. 10:1361–1368.39. Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001. 24:450–455.40. Leach MJ, Marden CM, Miller AA. Pharmacological studies of lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia. 1986. 27:490–497.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intrathecal lamotrigine blocks and reverses antinociceptive morphine tolerance in rats
- Effect of intrathecal oxcarbazepine on rat tail flick test-determined morphine tolerance
- Supraspinal Nitric Oxide Synthesis Inhibition Enhanced Antinociception of Morphine in Morphine Tolerant Rats
- The Effect of Intrathecal Epigallocatechin Gallate on the Development of Antinociceptive Tolerance to Morphine
- The role of group III metabotropic receptors on opioid tolerance