Korean J Physiol Pharmacol.
2012 Feb;16(1):11-16. 10.4196/kjpp.2012.16.1.11.
Effect of 5-FU and MTX on the Expression of Drug-resistance Related Cancer Stem Cell Markers in Non-small Cell Lung Cancer Cells
- Affiliations
-
- 1College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. hshin@konkuk.ac.kr
- KMID: 2285434
- DOI: http://doi.org/10.4196/kjpp.2012.16.1.11
Abstract
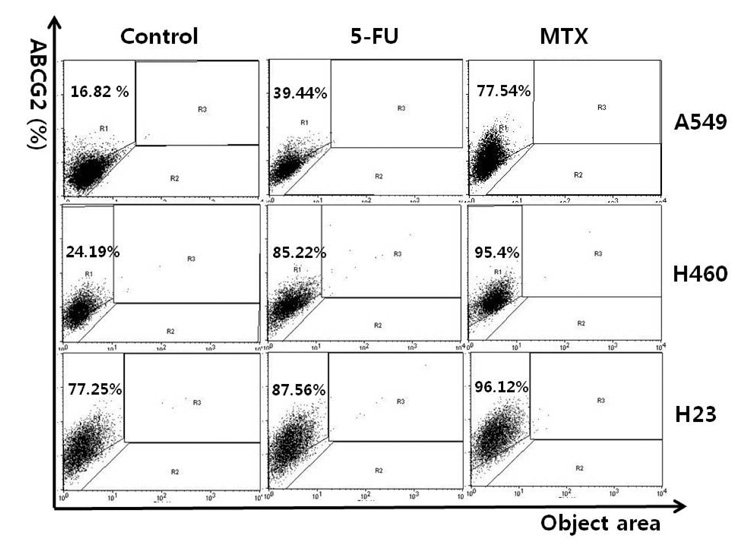
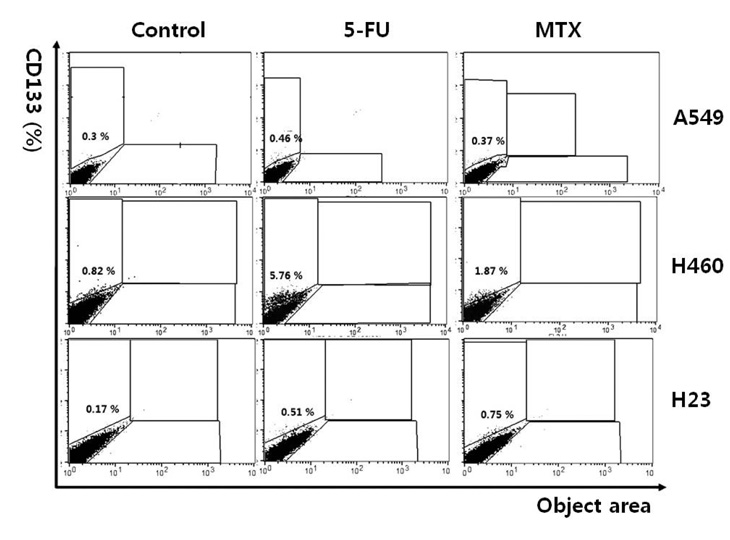
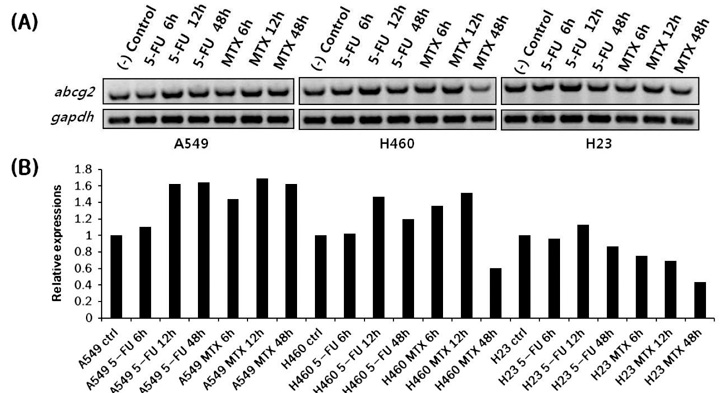
- Cancer stem cells (CSCs) are often characterized by the elevated expression of drug-resistance related stem-cell surface markers, such as CD133 and ABCG2. Recently, we reported that CSCs have a high level of expression of the IL-6 receptor (IL-6R). The purpose of this study was to investigate the effect of anticancer drugs on the expression of the drug resistance-related cancer stem cell markers, ABCG2, IL-6R, and CD133 in non-small cell lung cancer (NSCLC) cell lines. A549, H460, and H23 NSCLC cell lines were treated with the anticancer drugs 5-fluorouracil (5-FU; 25 microg/ml) and methotrexate (MTX; 50 microg/ml), and the expression of putative CSC markers was analyzed by fluorescent activated cell sorter (FACS) and the gene expression level of abcg2, il-6r and cd133 by reverse transcriptasepolymerase chain reaction (RT-PCR). We found that the fraction of ABCG2-positive(+) cells was significantly increased by treatment with both 5-FU and MTX in NSCLC cells, and the elevation of abcg2, il-6r and cd133 expressions in response to these drugs was also confirmed using RT-PCR. Also, the number of IL-6R(+) cells was increased by MTX in the 3 cell lines mentioned and increased by 5-FU in the H460 cell line. The number of CD133(+) cells was also significantly increased by both 5-FU and MTX treatment in all of the cell lines tested. These results indicate that 5-FU and MTX considerably enhance the expression of drug-resistance related CSC markers in NSCLC cell lines. Thus, we suggest that antimetabolite cancer drugs, such as 5-FU and MTX, can lead to the propagation of CSCs through altering the expression of CSC markers.
Keyword
MeSH Terms
Figure
Reference
-
1. Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005. 65:5506–5511.2. Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003. 3:895–902.3. Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabrò E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133(+) cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009. 106:16281–16286.4. Yang JP, Liu Y, Zhong W, Yu D, Wen LJ, Jin CS. Chemoresistance of CD133+ cancer stem cells in laryngeal carcinoma. Chin Med J (Engl). 2011. 124:1055–1060.5. Yanamoto S, Kawasaki G, Yamada S, Yoshitomi I, Kawano T, Yonezawa H, Rokutanda S, Naruse T, Umeda M. Isolation and characterization of cancer stem-like side population cells in human oral cancer cells. Oral Oncol. 2011. 47:855–860.6. Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997. 90:5013–5021.7. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004. 432:396–401.8. Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008. 68:9703–9711.9. Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007. 1:313–323.10. Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008. 15:504–514.11. Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008. 3:e3077.12. Stanley K, Stjernswärd J. Lung cancer--a worldwide health problem. Chest. 1989. 96:1 Suppl. 1S–5S.13. Jiang T, Collins BJ, Jin N, Watkins DN, Brock MV, Matsui W, Nelkin BD, Ball DW. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res. 2009. 69:845–854.14. Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, Ku HH, Chiou SH. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008. 3:e2637.15. Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabrò E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009. 106:16281–16286.16. Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010. 126:950–958.17. Seo DC, Sung JM, Cho HJ, Yi H, Seo KH, Choi IS, Kim DK, Kim JS, El-Aty AM A, Shin HC. Gene expression profiling of cancer stem cell in human lung adenocarcinoma A549 cells. Mol Cancer. 2007. 6:75.18. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005. 41:2502–2512.19. Tchirkov A, Khalil T, Chautard E, Mokhtari K, Véronèse L, Irthum B, Vago P, Kémény JL, Verrelle P. Interleukin-6 gene amplification and shortened survival in glioblastoma patients. Br J Cancer. 2007. 96:474–476.20. Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, Bromberg JF. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007. 117:3846–3856.21. Conze D, Weiss L, Regen PS, Bhushan A, Weaver D, Johnson P, Rincón M. Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res. 2001. 61:8851–8858.22. Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, Eyler CE, Elderbroom J, Gallagher J, Schuschu J, MacSwords J, Cao Y, McLendon RE, Wang XF, Hjelmeland AB, Rich JN. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009. 27:2393–2404.23. Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W, Liu Z. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem. 2010. 340:265–273.24. Campard D, Vasse M, Rose-John S, Poyer F, Lamacz M, Vannier JP. Multilevel regulation of IL-6R by IL-6-sIL-6R fusion protein according to the primitiveness of peripheral blood-derived CD133+ cells. Stem Cells. 2006. 24:1302–1314.25. Rice A, Barbot C, Lacombe F, Dubosc-Marchenay N, Marit G, Hau F, Boiron JM, Reiffers J. 5-fluorouracil permits access to a primitive subpopulation of peripheral blood stem cells. Stem Cells. 1993. 11:326–335.26. Bertolini F, Battaglia M, Soligo D, Corsini C, Curioni C, Lazzari L, Pedrazzoli P, Thalmeier K. "Stem cell candidates" purified by liquid culture in the presence of Steel factor, IL-3, and 5FU are strictly stroma-dependent and have myeloid, lymphoid, and megakaryocytic potential. Exp Hematol. 1997. 25:350–356.27. Todaro M, Perez Alea M, Scopelliti A, Medema JP, Stassi G. IL-4-mediated drug resistance in colon cancer stem cells. Cell Cycle. 2008. 7:309–313.28. Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009. 27:1006–1020.29. Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002. 62:5035–5040.30. Sung JM, Cho HJ, Yi H, Lee CH, Kim HS, Kim DK, Abd El-Aty AM, Kim JS, Landowski CP, Hediger MA, Shin HC. Characterization of a stem cell population in lung cancer A549 cells. Biochem Biophys Res Commun. 2008. 371:163–167.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD44 and CD133 as Cancer Stem Cell Markers for Gastric Cancer
- Urushiol V Suppresses Cell Proliferation and Enhances Antitumor Activity of 5-FU in Human Colon Cancer Cells by Downregulating FoxM1
- New Findings on Breast Cancer Stem Cells: A Review
- Differential Mucin Gene Expression Associated with Methotrexate Resistance of Human Colonic Adenocarcinoma Cell Line, HT29
- Effects of Combination Chemotherapy Depending on The Expression of Neuron Specific Enolase (NSE), P-Glycoprotein (PgP) and Glutathione S-Transferase (GST)-pai in Advanced Non-Small Cell Lung Cancer